Team:BYU Provo/Team Thermosensor/Week21
From 2011.igem.org

|
Contents |
September 6
- Plates T61-1, T61-3, T61-5 (30C) sectors 7&8 blue other sectors white
- Plates T61-2, T61-4, T61-6 (37C) sectors 7&8 blue while other sectors had hints of blue towards end of streaks and rest of the streak was white.
Set up more ligation mix
Lig 61-1/61-2 (recipe below x’s 2)
- 6.5 ul ddH20
- 1.5 ul 10x ligase buffer
- 1 ul DNA ligase
- 3ul insert DNA (55-1gs)
- 1ul vector (pIG15)
Set up and completed standard transformation
Plated Lig 61-1 and 61-2
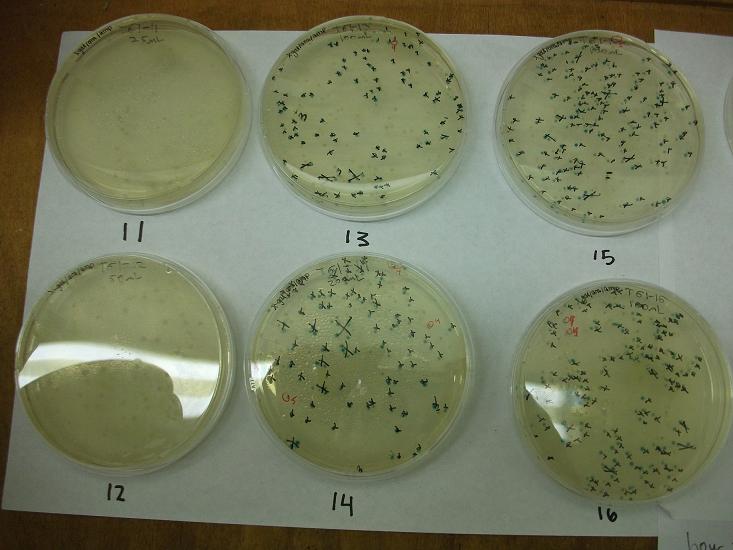
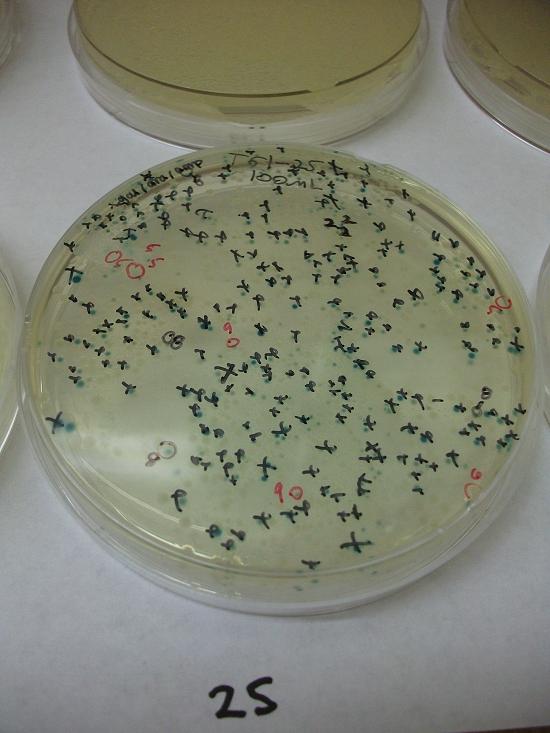
- Plates T61-11 through plates T61-19 plated with ligation 61-1 with 25, 50, 100, 200, 100(plates 15-19) mL respectively
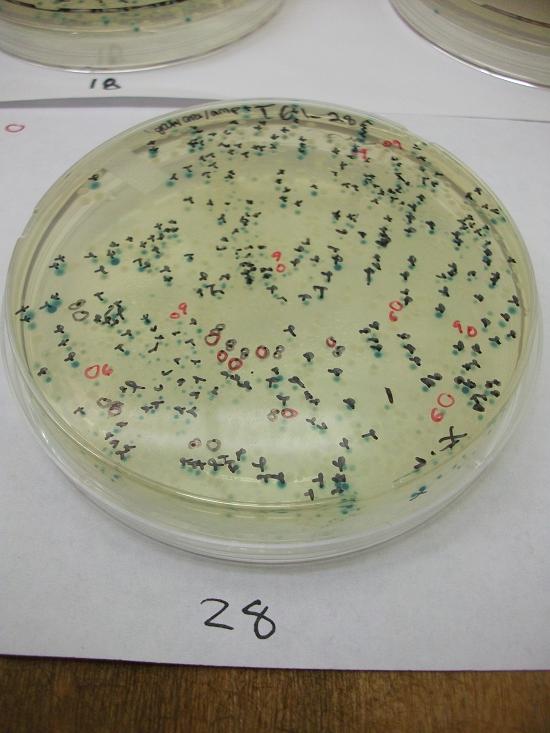
- Plates T61-21 through plates T61-29 plated with ligation 61-2 with 25, 50, 100, 200, 100(plates 25-29) mL respectively.
- All plates plated at 30C except T61-19 which was plated at 37C
Septermber 7
Took 37C plate out—Lots of variation in LacZ expression 30oC plates will be left in for an additional day to grow larger colonies
September 8
Thermosensor Test
Plates T61-11 through T61-29 (except plate T61-19, the plate that was originally put at 37C) moved from 30C to the 37C incubator at 8:00am. Marked all blue colonies with an “x” so that we can distinguish those that were initially blue from those which change expression of LacZ (thermosensor activitiy) and go from white to blue. Used plate T61-19 which had been placed at 37C and had variation in LacZ expression to define what we would call blue once changed. When initially transferred, some Plates were more than 50% blue at 30C We went through hourly and marked colonies that turned blue with the respective hour in which they were checked and appeared blue.
September 9
Dr. Grose, and ourselves for that matter, are very excited about how this experiment and how varied these colonies turned out to be. Next we need to streak the colonies that changed after the experiment (any of the colonies labeled with a number). We will put these at 30C and then watch them at 37C to ensure that they actually do change about the same time again. Those colonies that are still lighter in blue color we will transfer to 42C and observe their change in color, hopefully a dark blue. We also need the wild-type Thermosensor A as a reference on multiple plates. We need to keep track again at what hour they all changed.
Ligation (63-1)
- Standard protocol except doubled and 6ul insert DNA (gs 55-2, mutagenic thermosensors) and 2ul pIG15.
Transformation
- Standard protocnol except incubating cells in 1mL of LB instead of 0.5mL.
- Plated 75ul cells, all
Plates 63-2 through 63-11 left at room temperature 9/9, 9/10, put at 30C 9/11 Plates 63-11 through 63-20 left at room temperature 9/9, 9/10, put at 37 9/11 Plates 63-21 and 63-22 same as 2-11 but will put them at 37/42oC for ~1hr. then remove and let rest at room temperature ~4hrs. Observe Plates 23-24 will be left at room temperature 9/9, 9/10, 9/11, 9/12 then 23 will be put at 30C. Observe after several hours. 24 will be put at 37C—observe after several hours
 "
"