Team:BYU Provo/Team OxyR/Week2
From 2011.igem.org
(→28 April 2011) |
|||
| Line 32: | Line 32: | ||
Even the positive control (far right) was fuzzy. We don't know how well it worked. | Even the positive control (far right) was fuzzy. We don't know how well it worked. | ||
| - | + | ==29 April 2011== | |
| + | We tried the ligation again, to clone the KatG and HemH promoters in front of GFP. Dr. Grose thinks that even though the PCR looks fuzzy, they probably worked, so the restriction digested fragments came from that PCR run. Dr. Grose suggested re-digesting the pPlat/GFP plasmid again, if this ligation doesn't work. | ||
</div> | </div> | ||
Latest revision as of 23:22, 27 September 2011

|
Contents |
25 April 2011
We purified the most recent cloning: pPlat that contained the directionally cloned GFP.
Also, purified the PCR amplification of KatG and HemH from April 19th. By referring to KatG and HemH we are actually referring to the promoters "PHemH and PKatG".
26 April 2011
Rob and Mackay ran the restriction digest for the HemH and KatG inserts, as well as the plasmid backbone.
Matt came in later and ran the low-melt gel. The digest worked great and we stored the slices in the -20.
27 April 2011
Matt came in and set up ligations with the fragments from April 26th. If this ligation works, we should end up with a plasmid that contains GFP under the control of an OxyR-regulated promoter. One version will have KatG and the other version will have HemH.
28 April 2011
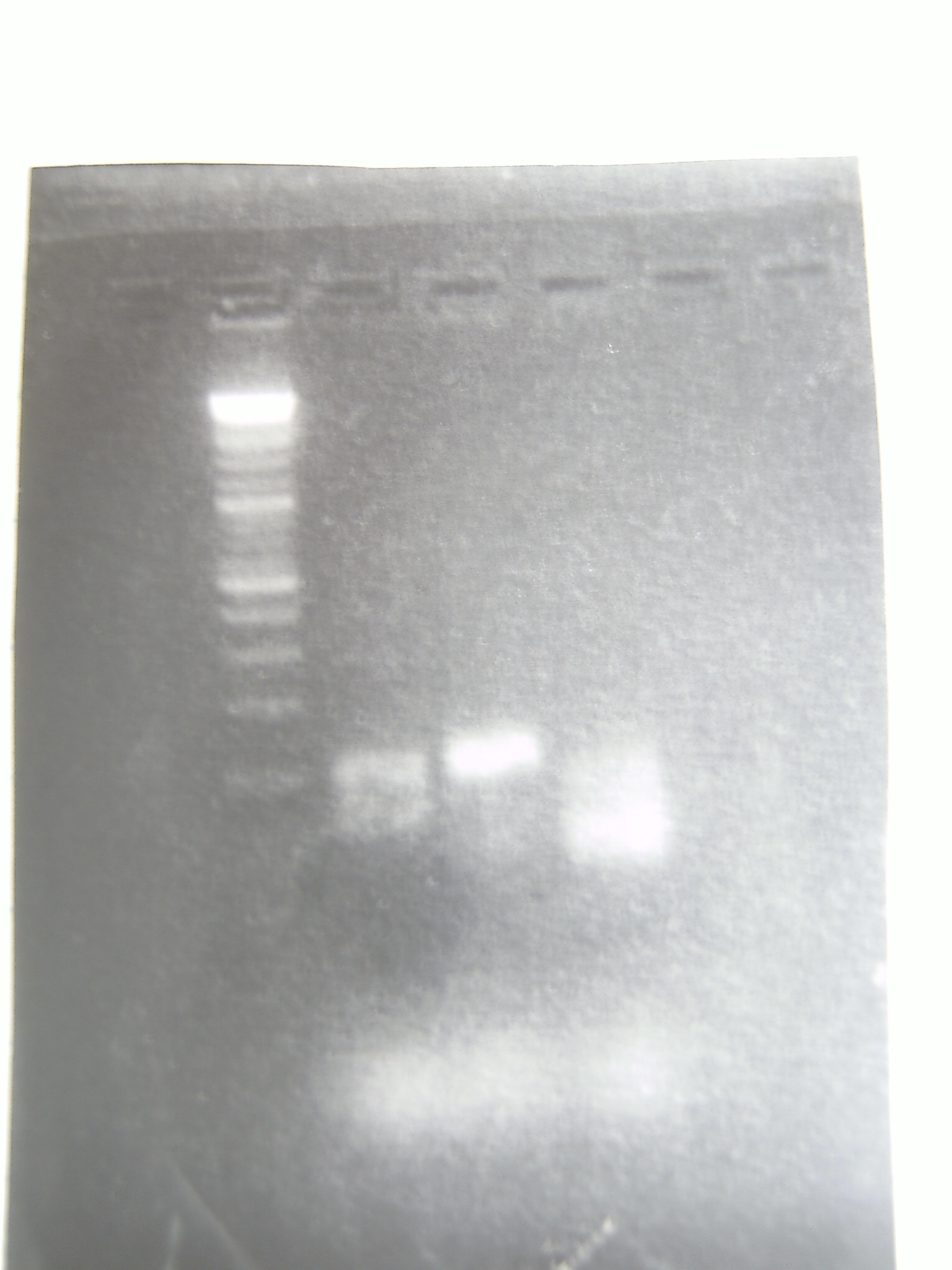
Neither ligation worked. Not a single colony appeared. We think it may have been a problem with the inserts, so we tried re-amplifying the inserts. More carefully this time.
Even the positive control (far right) was fuzzy. We don't know how well it worked.
29 April 2011
We tried the ligation again, to clone the KatG and HemH promoters in front of GFP. Dr. Grose thinks that even though the PCR looks fuzzy, they probably worked, so the restriction digested fragments came from that PCR run. Dr. Grose suggested re-digesting the pPlat/GFP plasmid again, if this ligation doesn't work.
 "
"