Team:BYU Provo/Team OxyR/Week19
From 2011.igem.org
(→16 August 2011) |
(→18 August 2011) |
||
| Line 24: | Line 24: | ||
==18 August 2011== | ==18 August 2011== | ||
Minipreps gave decent results. The get was made from old, slimy buffer, and looked a bit rough, but at least confirmed that plasmid was indeed purified. The miniprep for the low-copy plasmid was a bit more involved because two columns were run in parallel, with double the pellet size, to quadruple the amount of plasmid DNA in the final elution stage. | Minipreps gave decent results. The get was made from old, slimy buffer, and looked a bit rough, but at least confirmed that plasmid was indeed purified. The miniprep for the low-copy plasmid was a bit more involved because two columns were run in parallel, with double the pellet size, to quadruple the amount of plasmid DNA in the final elution stage. | ||
| - | + | ||
| + | [[File:GelPic18Aug.JPG|350px|thumb|left|Gel Image 18 August 2011]] | ||
| + | |||
Restriction digests were set up with the pre-determined enzymes. Predicting the fragment length took a bit of 3x5 card calculating to figure out, but the predicted gel was almost spot on. When the digests were run on the low-melt gel, the plasmids with oxyR in them didn't resolve into separate bands because the length of the backbone was about the same as the insert. Both were cut out together. | Restriction digests were set up with the pre-determined enzymes. Predicting the fragment length took a bit of 3x5 card calculating to figure out, but the predicted gel was almost spot on. When the digests were run on the low-melt gel, the plasmids with oxyR in them didn't resolve into separate bands because the length of the backbone was about the same as the insert. Both were cut out together. | ||
Revision as of 05:24, 14 September 2011

|
Contents |
15 August 2011
Matt made another to-do list. "Streak OxyR- to singles for electroporation, set up overnight cultures for plasmid preparations, etc."
Mackay, minipreping.
16 August 2011
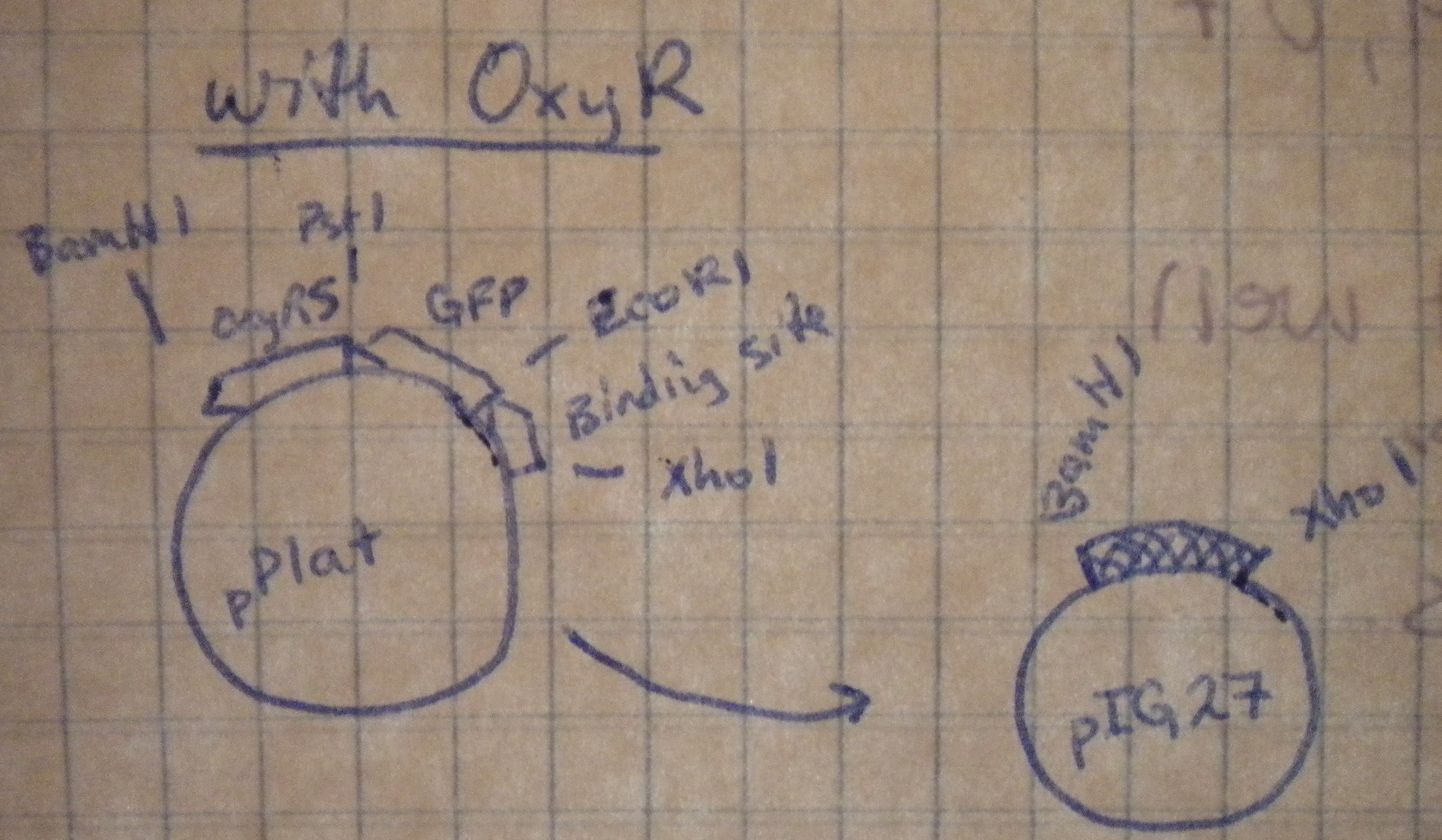
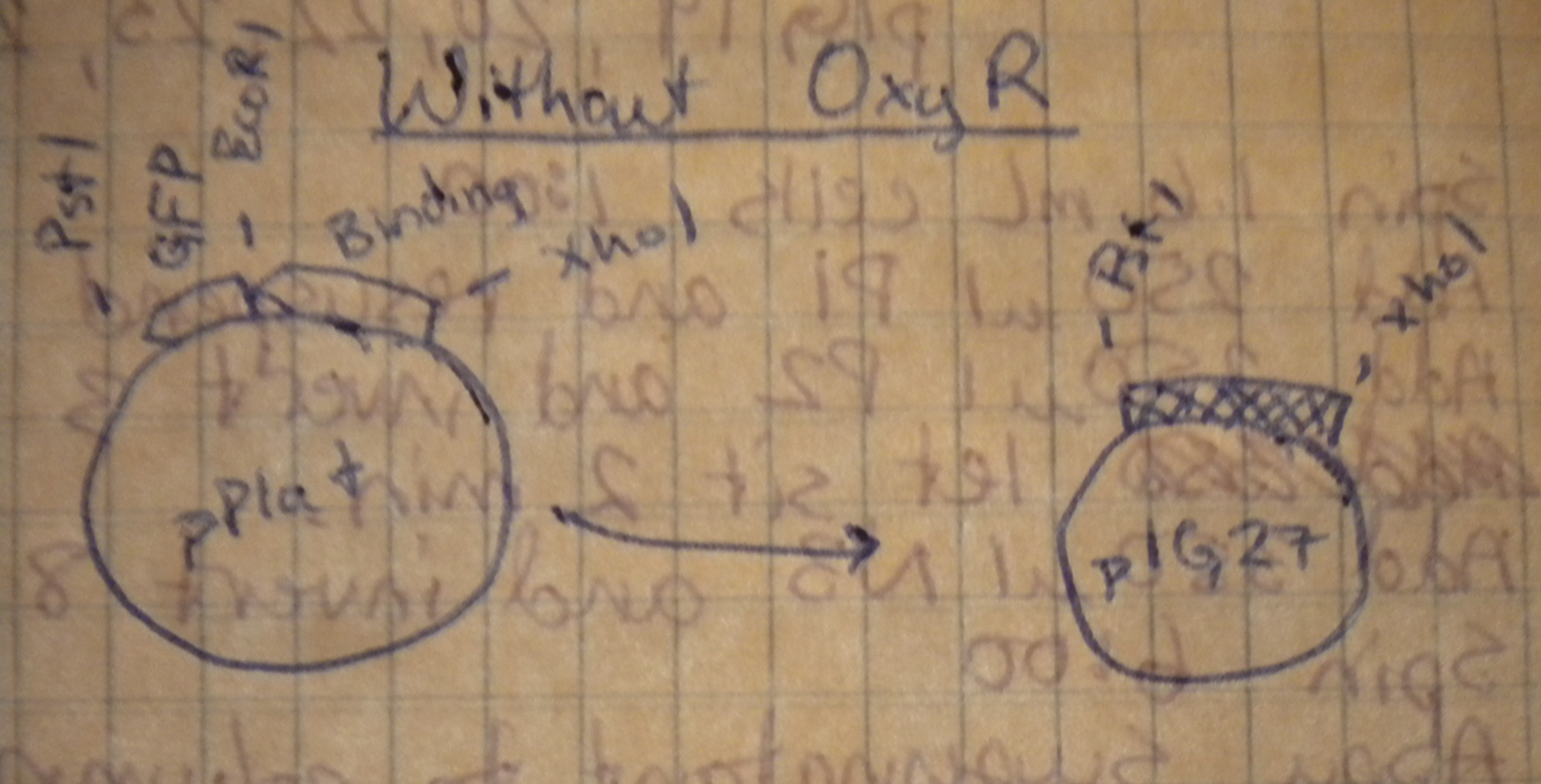
Prepared overnight cultures, and planned restriction digests to transfer our multiple cloning sites from the high-copy pPlat into the iGEM low-copy pSB4A5. Notebook diagrams helped determine the appropriate restriction enzymes.
Those with oxyR on the plasmid would be cut with XhoI and BamHI, while those without oxyR would be cut with XhoI and PstI.
17 August 2011
Overnight cultures were set up with all the constructs to-date, prepared for Plasmid minipreps the next day.
18 August 2011
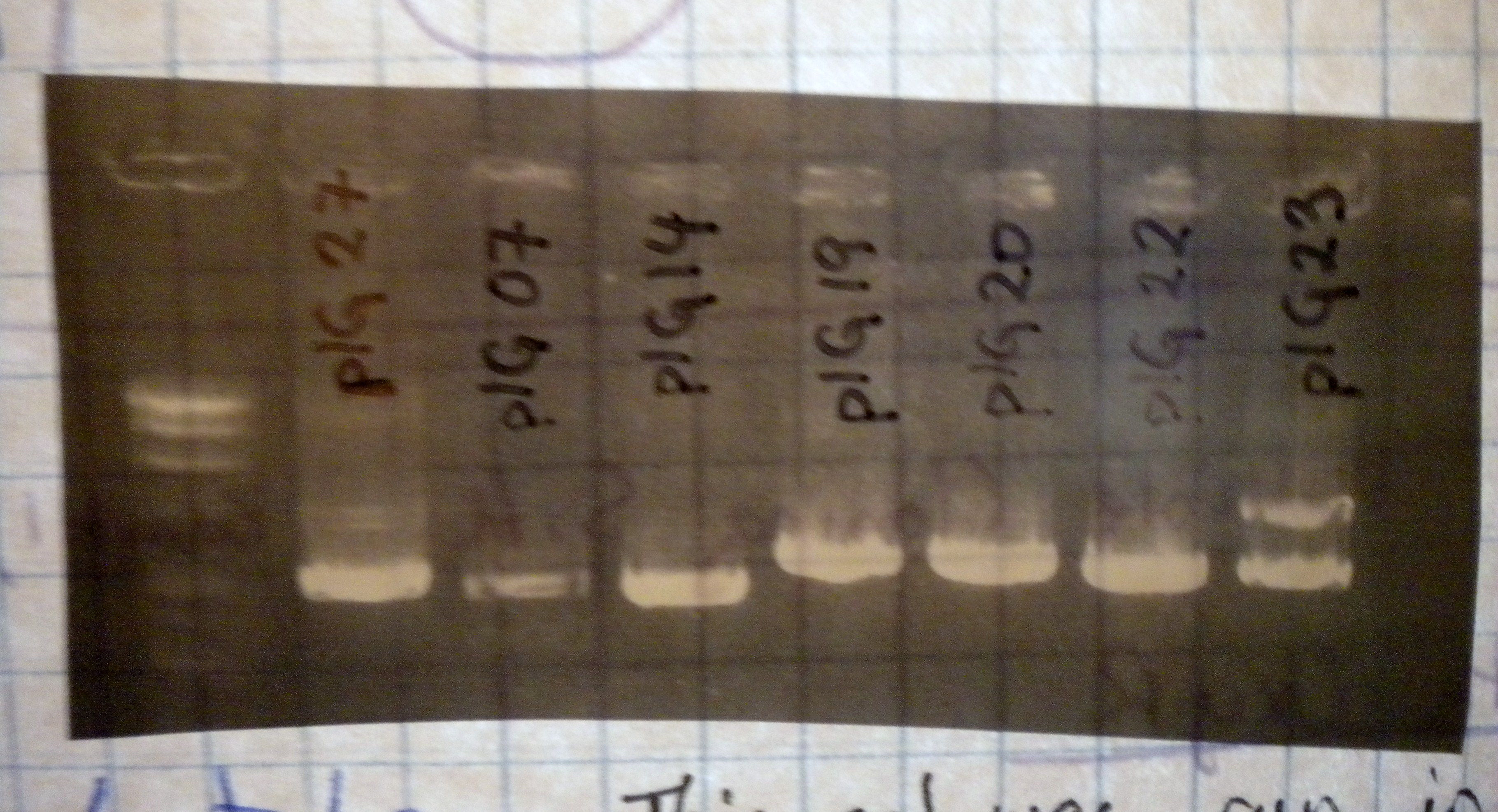
Minipreps gave decent results. The get was made from old, slimy buffer, and looked a bit rough, but at least confirmed that plasmid was indeed purified. The miniprep for the low-copy plasmid was a bit more involved because two columns were run in parallel, with double the pellet size, to quadruple the amount of plasmid DNA in the final elution stage.
Restriction digests were set up with the pre-determined enzymes. Predicting the fragment length took a bit of 3x5 card calculating to figure out, but the predicted gel was almost spot on. When the digests were run on the low-melt gel, the plasmids with oxyR in them didn't resolve into separate bands because the length of the backbone was about the same as the insert. Both were cut out together. "
"