Team:BYU Provo/Team OxyR/Week21
From 2011.igem.org
(→10 September 2011) |
|||
| (2 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:BYU_Provo}} | {{Template:BYU_Provo}} | ||
<html><head><meta http-equiv="X-UA-Compatible" content="IE=EmulateIE7"></head></html> | <html><head><meta http-equiv="X-UA-Compatible" content="IE=EmulateIE7"></head></html> | ||
| - | |||
<div id='pageContents'> | <div id='pageContents'> | ||
| - | + | <html><script> | |
| + | var ele = document.getElementById('liOxyR21'); | ||
| + | ele.style.fontWeight = "bold"; | ||
| + | ele.style.backgroundColor = "#059469"; | ||
| + | jQuery(document).ready(function(){ | ||
| + | $('.accordion').next().show(); | ||
| + | $('#OxyRList').show(); | ||
| + | }); | ||
| + | </script></html> | ||
==6 September 2011== | ==6 September 2011== | ||
| Line 10: | Line 17: | ||
==9 September 2011== | ==9 September 2011== | ||
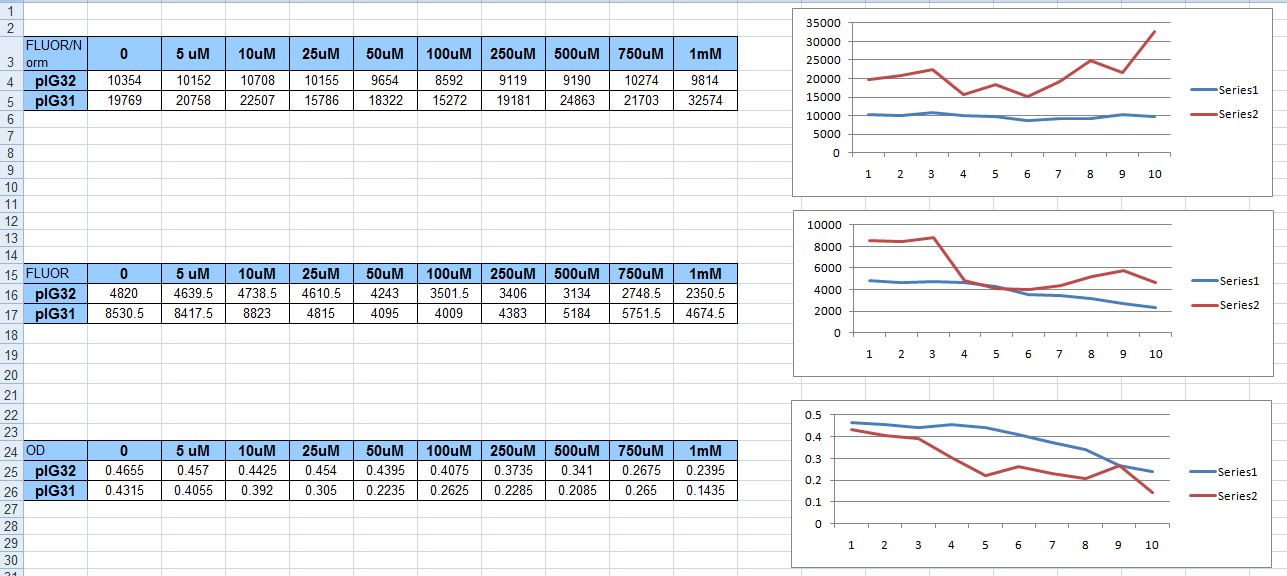
| + | We tried inducing just two strains this time, with ten concentrations of H2O2, instead of five. We covered the span depicted in the table below: 0uM on up to 1mM. We used the plasmids that had only the katG promoter fused to GFP, and only the hemH promoter fused to GFP; pIG31 and pIG32 respectively. As can be seen, the results were less than satisfactory. The random distribution of fluorescence never increased even two-fold. | ||
[[File:9_Sept_results_pic.JPG|350px|thumb|center|Plate Reader Experiment Results 9 September 2011]] | [[File:9_Sept_results_pic.JPG|350px|thumb|center|Plate Reader Experiment Results 9 September 2011]] | ||
| Line 15: | Line 23: | ||
==10 September 2011== | ==10 September 2011== | ||
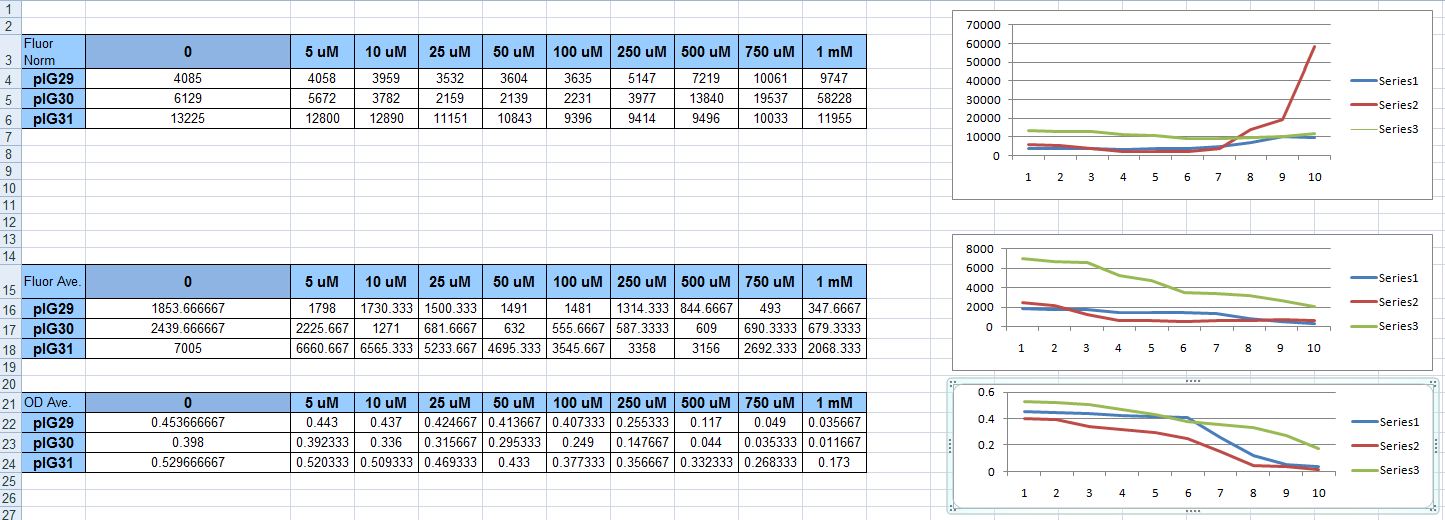
| + | We attempted the protocol again, only this time with the plasmid that had always given out-of-the-ordinary results before: pIG30 (hemH promoter fused to GFP, with the oxyR gene on the plasmid under a strong constitutive promoter). It didn't fail to look great on the graph. Unfortunately, that was because the cells were dying. Because we normalize fluorescence over OD, with the tiny OD's as H2O2 concentration rose, the normalized fluorescence also rose. It was misleading, and unfortunate. Not a single one of our constructs had proven to be a useful sensor. | ||
[[File:10_Sept_results_pic.JPG|350px|thumb|center|Plate Reader Experiment Results 10 September 2011]] | [[File:10_Sept_results_pic.JPG|350px|thumb|center|Plate Reader Experiment Results 10 September 2011]] | ||
| + | |||
| + | Moving forward, we've decided to take one more swing at it with an enlarged version of the katG promoter that is closer the version used in [http://www.springerlink.com/content/61pgne3fmraepylw/ the literature]. Just to be sure, we're planning the final construct with Pbad to demonstrate the AND gate action, in case the neo-katG doesn't work out. | ||
Latest revision as of 20:47, 27 September 2011

|
6 September 2011
Matt met with Dr. Grose and talked about the data from Friday's experiment. She felt that, once again, the huge peak was due to cell death. Maybe we were using too much H2O2. We planned to revise the protocol and try again.
9 September 2011
We tried inducing just two strains this time, with ten concentrations of H2O2, instead of five. We covered the span depicted in the table below: 0uM on up to 1mM. We used the plasmids that had only the katG promoter fused to GFP, and only the hemH promoter fused to GFP; pIG31 and pIG32 respectively. As can be seen, the results were less than satisfactory. The random distribution of fluorescence never increased even two-fold.
10 September 2011
We attempted the protocol again, only this time with the plasmid that had always given out-of-the-ordinary results before: pIG30 (hemH promoter fused to GFP, with the oxyR gene on the plasmid under a strong constitutive promoter). It didn't fail to look great on the graph. Unfortunately, that was because the cells were dying. Because we normalize fluorescence over OD, with the tiny OD's as H2O2 concentration rose, the normalized fluorescence also rose. It was misleading, and unfortunate. Not a single one of our constructs had proven to be a useful sensor.
Moving forward, we've decided to take one more swing at it with an enlarged version of the katG promoter that is closer the version used in [http://www.springerlink.com/content/61pgne3fmraepylw/ the literature]. Just to be sure, we're planning the final construct with Pbad to demonstrate the AND gate action, in case the neo-katG doesn't work out. "
"