Team:BYU Provo/Results
From 2011.igem.org

|
Results
Thermosensor Results The following table summarizes the results of the random mutagenesis on the Lysteria thermosensor. We found two thermosensors with narrower temperature ranges, and four thermosensors with increased or decreased expression after melting. We are especially pleased with the narrowed temperature ranges of BBa_K619890 and BBa_K619891, which demonstrate the most narrow temperature sensitivity of any known riboswitches from our literature research.
| Part Number | Melting Range |
|---|---|
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619889 BBa_K619889] | 30'C - 37'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619890 BBa_K619890] | 35'C - 37'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619891 BBa_K619891] | 30'C - 35'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619892 BBa_K619892] | 30'C - 37'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619893 BBa_K619893] | 30'C - 37'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619894 BBa_K619894] | 30'C - 37'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619895 BBa_K619895] | 30'C - 37'C |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619897 BBa_K619897] | Positive Control |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K619898 BBa_K619898] | Positive Control |
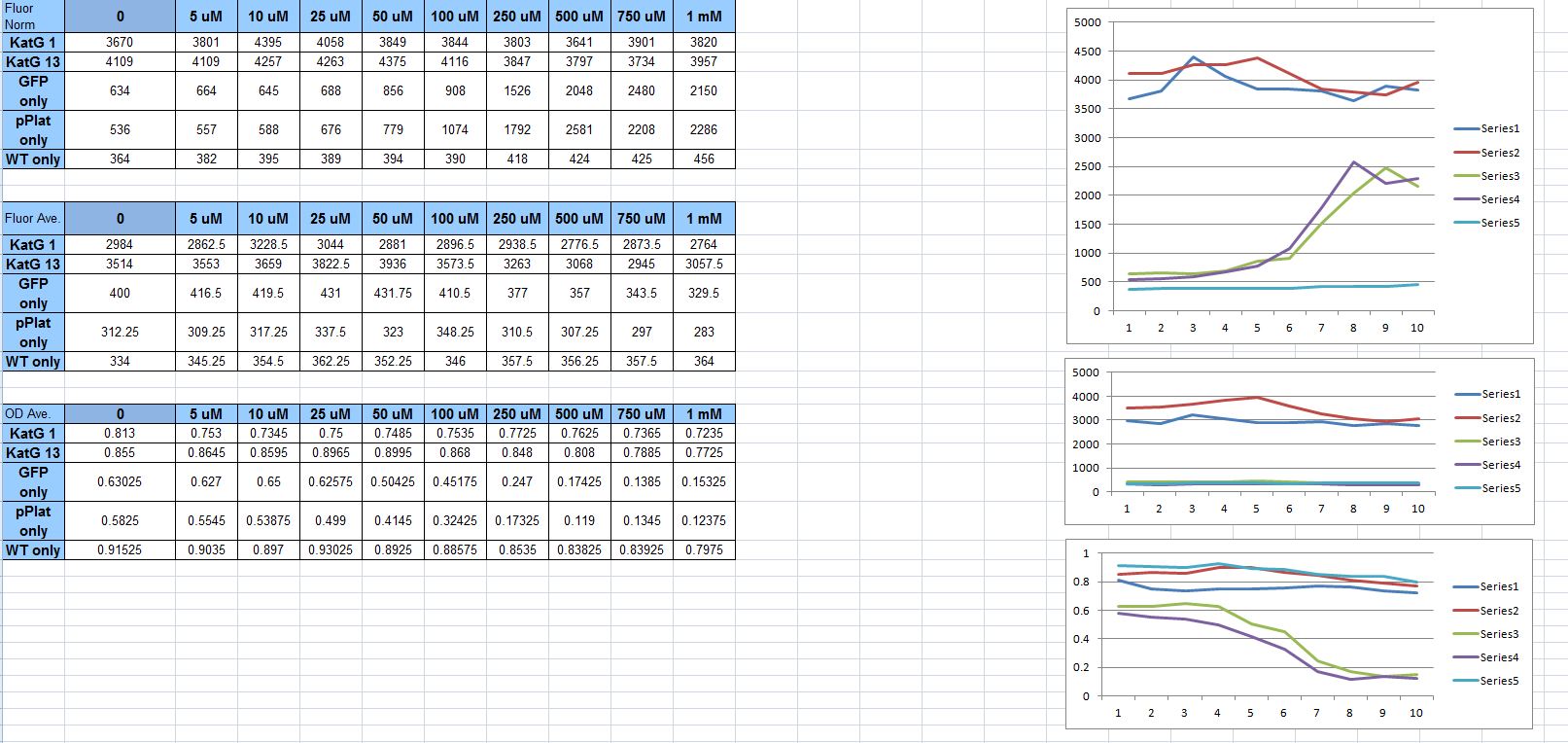
OxyR Promoter Results Our OxyR results were inconclusive. Despite documentation in the literature about the reactive oxygen species (ROS)-inducible promoters katG, hemH and soxS, we were unable to produce consistent results demonstrating their usefulness as ROS sensors. The results of our final assay can be seen in the graphs below:
5 hour readings
The graphs represent (from bottom to top) the OD, the raw fluorescence, and the normalized fluorescence (fluorescence/OD). The clustered lines on the bottom of the graphs are the negative controls (empty plasmid, promoter-less GFP) and a positive control for fluorescence. The top lines are the final versions of our katG promoters controlling GFP expression. The horizontal axis is ROS concentration, which increases from left to right.
As can be seen, there is no significant increase in fluorescence with increased hydrogen peroxide concentration.
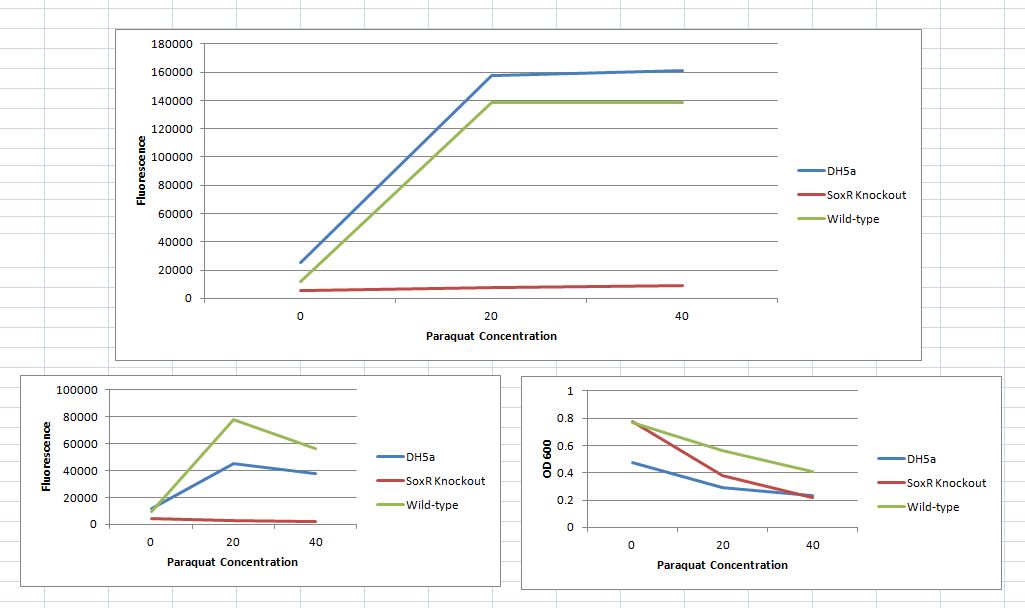
SoxS Promoter Results Over the summer, we built an ROS sensor using the SoxR promoter which we never tested with paraquat. After Indiana, we came home and tested it using paraquat, with great results. Unlike team UNICAMP-EMSE Brazil, our device doesn't include the SoxR protein, expressed under a constitutive promoter, but works just fine with the natural levels of SoxR present in the wild-type E. coli strain we used (K12). As can be seen, there is some basal fluorescence in the SoxR knockout strain, but it is obvious that SoxR is the major activating factor in this super-oxide sensor. Also, unlike the UNICAMP_EMSE team, our sensor reached peak fluorescence at 20 uM paraquat concentrations, rather than 40 uM.
Each sample was run in triplicate, and the averaged ODs and fluorescence values are displayed in the graph. The raw OD and fluorescence are shown below, and the normalized fluorescence (Fluorescence / OD) is shown above. These data points were taken 3.5 hours after inducing with paraquat.
We are very pleased that the soxS promoter works as a super-oxide sensor without producing extra SoxR protein within the cell. It is also interesting to note that with this construct, the fluorescence peaks at 20 uM rather than 40 uM of paraquat.
Overall Results The AND gate function was very successful when using pBAD in place of the OxyR-inducible promoters. pBAD worked as an "arabinose sensor" which demonstrated proof of concept. We were very pleased with the range of thermosensors we produced. The variety offers flexibility to future users.
 "
"