Team:UNICAMP-EMSE Brazil/Notebook/27 August 2011
From 2011.igem.org

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |
Contents |
Notebook
Click on a date to see what we have done!
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
27 August 2011
Purification of SoxS and RBS+QseB+RBS+Qsec+T and Quantification of 26/Aug/2011 samples:
Objective:
- Purification of digested SoxS and RBS+QseB+RBS+Qsec+T and quantification of the purification performed yesterday of digested samples.
Samples: GEL 1
- SoxS (S/P) / SoxS (S/P)/ SoxS (S/P)/ RBS+QseB+RBS+Qsec+T (X/P)/ RBS+QseB+RBS+Qsec+T (X/P)/ RBS+QseB+RBS+Qsec+T (X/P)/ HlyA C2/ RBS+SoxR+T/RBS+IL 12/ RBS+HlyB/ RBS+GFP/HlyA C1/ RBS+IL 10/fLHDC/Constitutive promoter.
- Ladder:
- Bio-Rad 100bp – 10.000bp
- Gel Agarose concentration:
- 1%
OBS:
- S/P: digested with Spe and Pst
- X/P: digested with Xba and Pst
- Results:
- Only HlyA was not detected by the gel. Problems with this molecule purification from the gel.
Quantification:
Objective:
- Quantification of digested SoxS and RBS+QseB+RBS+Qsec+T purified from the former gel.
- Samples: GEL 2
- SoxS / RBS+QseB+RBS+Qsec+T
- Ladder:
- Bio-Rad 100bp – 10.000bp
- Gel Agarose concentration:
- 1,5%
- Results:
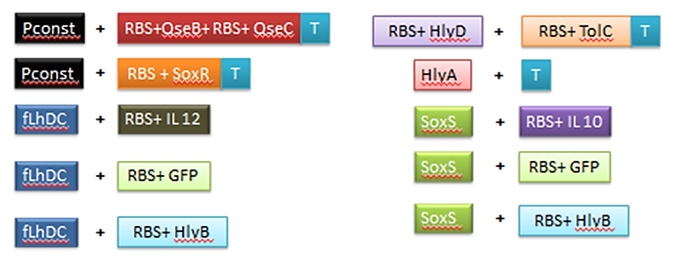
Good bands! Lets ligate them following the schema below:
Digestions recipes
- RBS+IL-10 - SoxS_promoter(vector)
- 9 ul - milli-Q water
- 7 ul - RBS+IL-10 (~60ng)
- 1 ul - SoxS_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
- RBS+GFP - SoxS_promoter(vector)
- 6 ul - milli-Q water
- 10 ul - RBS+GFP (~60ng)
- 1 ul - SoxS_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
- RBS+HlyB - SoxS_promoter(vector)
- 14 ul - milli-Q water
- 2 ul - RBS+HlyB (~50ng)
- 1 ul - SoxS_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
- RBS+QseB+RBS+QseC+Term - Constitutive_promoter(vector)
- 11 ul - milli-Q water
- 1 ul - RBS+QseB+RBS+QseC+Term (~50ng)
- 5 ul - Constitutive_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
After all digestions (from 26/08/2011): lIGATIONS
- HlyA - terminator(vector)
- 3 ul - milli-Q water
- 30.5 ul - HlyA (?ng)
- 1ul - terminator(vector) (~30ng)
- 4ul - 10X T4 Buffer
- 1.5ul - T4 DNA Ligase
- TOTAL = 40ul
- RBS+IL-12 - flhDC_promoter(vector)
- 11.5 ul - milli-Q water
- 4 ul - RBS+IL-12 (~60ng)
- 1.5 ul - flhDC_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
- RBS+SoxR+Term - Constitutive_promoter(vector)
- 8.5 ul - milli-Q water
- 3.5 ul - RBS+SoxR+Term (~60ng)
- 5 ul - Constitutive_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
- RBS+GFP - flhDC_promoter(vector)
- 5.5 ul - milli-Q water
- 10 ul - RBS+GFP (~60ng)
- 1.5 ul - flhDC_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
- RBS+HlyB - flhDC_promoter(vector)
- 13.5 ul - milli-Q water
- 2 ul - RBS+HlyB (~50ng)
- 1.5 ul - flhDC_promoter(vector) (~20ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
Tasks:
Incubate all ligation reactions for 1-2 hour at 22°C.
Use 5ul of these ligations to do the transformation (except for HlyA - 20ul)

 "
"