Team:UNICAMP-EMSE Brazil/Notebook/22 August 2011
From 2011.igem.org

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |
Contents |
Notebook
Click on a date to see what we have done!
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
22 August 2011
Digestion of our synthetic genes
Objective: Cut the band of the opened vector (terminator) or cut gene (all the rest);
- Ladder: 100 bp Bio-Rad
- Samples:
- (all digested, forgot to apply the closed vector sample in the gel) Terminator/ Terminator/ RBS+GFP/ RBS+GFP/ RBS+HlyD/ RBS+HlyB/ RBS+TolC/ HlyA/ RBS+SoxR/ RBS+QseB+RBS+QseC
- Results:
| Gene | Total size (gene+vector) | Linear vector size | Gene size | Result |
|---|---|---|---|---|
| Terminator | ~3300 pb | 3300 pb | No cut | Ok, complete digestion |
| RBS+GFP | ~2800 pb | 2079 pb | 735 pb | Ok, complete digestion |
| RBS+HlyD | 4296 pb | 2800 pb | 1496 pb | Ok, complete digestion |
| RBS+HlyB | 4983 pb | 2800 pb | 2183 pb | Parcial digestion |
| RBS+TolC | 4380 pb | 2800 pb | 1580 pb | Ok, complete digestion |
| HlyA | 3027 pb | 2800 pb | 227 pb | Ok, complete digestion |
| RBS+SoxR | 3323 pb | 2800 pb | 523 pb | Ok, complete digestion |
| RBS+QseB+RBS+QseC | 4886 pb | 2800 pb | 2086 pb | Parcial digestion |
All the genes were purified using Fermentas kit (25 uL elution) and quantified through electrophoresis:
- Ladder: 100 bp Bio-Rad
- Objective:
- Quantification (volume of ladder applied 6 uL; sample: 5 uL DNA +1 uL buffer )
- Samples: Terminator/ Terminator / RBS+GFP/ RBS+GFP / RBS+HlyD/ RBS+HlyB/ RBS+TolC/ HlyA /RBS+SoxR /RBS+QseB+RBS+QseC
- HlyA did not appear in the gel!! Digest and purify again !!!
Nd: non digested
D: digested
D: digested
Promoter testings
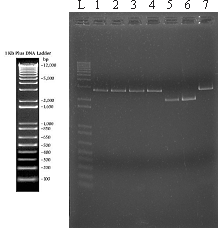
Third Electrophoresis Gel, 22/08/2011.
Gel 2: L = 1Kb Plus DNA Ladder Invitrogen
1 = SoxS promoter - 100ng of the synthesized construction
2 = flhDC promoter - 100ng of the synthesized construction
3 and 4 = SoxS promoter - 2ul of the miniprep
5 and 6 = Constitutive promoter - 2ul of the miniprep
7 = flhDC promoter - 2ul of the miniprep.
Gel 2: L = 1Kb Plus DNA Ladder Invitrogen
1 = SoxS promoter - 100ng of the synthesized construction
2 = flhDC promoter - 100ng of the synthesized construction
3 and 4 = SoxS promoter - 2ul of the miniprep
5 and 6 = Constitutive promoter - 2ul of the miniprep
7 = flhDC promoter - 2ul of the miniprep.
- Objective:
- Check the inserted promoters in our vectors
- Agarose gel: 1.5% stained with ethidium bromide
- Samples:
- L = 1Kb Plus DNA Ladder Invitrogen
- 1 = SoxS promoter - 100ng of the synthesized construction
- 2 = flhDC promoter - 100ng of the synthesized construction
- 3 and 4 = SoxS promoter - 2ul of the miniprep
- 5 and 6 = Constitutive promoter - 2ul of the miniprep
- 7 = flhDC promoter - 2ul of the miniprep
- Results and explanation
- Again, we did not observe any band corresponding to the excised insert. Since the vectors in which the synthesized promoters are cloned have no restriction sites for the enzymes we used (EcoRI and PstI), and given that we are observing linearized vectors after the digestions, we can conclude that there is at least something (an insert) in the vector that is being cut. So, we have two possibilities:
- (1) the amount of DNA in the excised insert is too low for visualizing in the agarose gel (which is possible in the case of very short DNA fragments);
- or (2) there is a mutation in the sequence recognized by one of the restriction enzymes, possibly inserted during the synthesis process (which is very unlikely to be occurring solely in the promoter sequences).

 "
"

