Team:UNICAMP-EMSE Brazil/Notebook/26 August 2011
From 2011.igem.org

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |
Contents |
Notebook
Click on a date to see what we have done!
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
26 August 2011
RBS+QseB+RBS+QseC+T (E-P) and SoxS
Objective:
- Checking of RBS+QseB+RBS+QseC+T (E-P) and SoxS (linearized with EcoRI) colonies from transformation
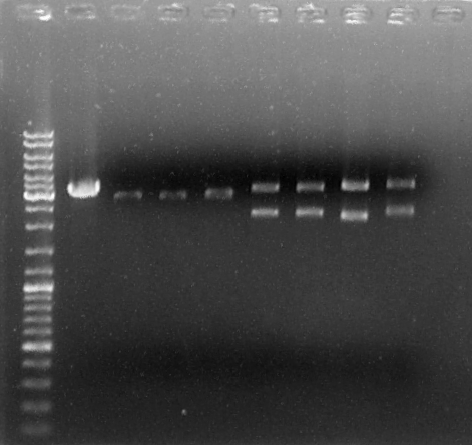
Samples: GEL 1
- Terminator 1 (checking if it is the Terminator vector linearized)/ SoxS C7/8 (d)/ SoxS c7/8 (d)/ SoxS C9 (d)/ RBS+QseB+RBS+Qsec+T C1 (d)/ RBS+QseB+RBS+Qsec+T C2 (d)/ RBS+QseB+RBS+Qsec+T C3 (d)/ RBS+QseB+RBS+Qsec+T C4 (d).
- Ladder:
- Bio-Rad 100bp – 10.000bp
- Gel Agarose concentration:
- 1%
OBS:
- D=digested
- Nd= non-digested
- Results:
| Gene | Total size (gene+vector) | Linear vector size | Gene size | Result |
|---|---|---|---|---|
| Terminator 1 | ~3300 pb | 3800 pb | No cut | OK |
| SoxS C7/8 | ~2900 pb | 2800 pb | No cut | OK |
| SoxS c7/8 | ~2900 pb | 2800 pb | No cut | OK |
| SoxS C9 | ~2900 pb | 2800 pb | No cut | OK |
| RBS+QseB+RBS+Qsec+T C1 | 4981 pb | 2800 pb | 2181 pb | OK |
| RBS+QseB+RBS+Qsec+T C2 | 4981 pb | 2800 pb | 2181 pb | OK |
| RBS+QseB+RBS+Qsec+T C3 | 4981 pb | 2800 pb | 2181 pb | OK |
| RBS+QseB+RBS+Qsec+T C4 | 4981 pb | 2800 pb | 2181 pb | OK |
Samples RBS+QseB+RBS+Qsec+T C1 and SoxS C7/8 were chosen to be digested for further purification and ligation.
Digestion and purification of genes
Objective:
- Digestion and purification of genes for further ligation reaction.
- Samples: GEL 2
- HlyA C1/HlyA C2/ FlhDC /constitutive promotor/ RBS+HlyB/RBS+SoxR+T/RBS+IL-12/ RBS+IL 10/RBS+GFP
- Ladder:
- Bio-Rad 100bp – 10.000bp
- Gel Agarose concentration:
- 1,5%
- Results:
| Gene | Total size (gene+vector) | Linear vector size | Gene size | Result |
|---|---|---|---|---|
| HlyA C1 | 3027 pb | 2800 pb | 277 pb | Ok, complete digestion |
| HlyA C2 | 3027 pb | 2800 pb | 227 pb | Ok, complete digestion |
| flHDC promoter | ~2926 pb | 2926 pb | No cut | Ok, linearization complete |
| RBS+HlyB | 4983 pb | 2800 pb | 2183 pb | Ok, problably mRFP cut out |
| RBS+SoxR+Terminator | ~3823 pb | 3300 pb | 618 pb | Ok, parcial digestion, no linear vector detect |
| RBS+IL12 | 4458 pb | 2800 pb | 1658 pb | Ok, complete digestion |
| RBS+IL10 | 3345 pb | 2800 pb | 545 pb | Ok, complete digestion |
| RBS+GFP | 2814 pb | 2079 pb | 735 pb | Ok, complete digestion |
Digestions recipes for checking the miniprep:
- vector+SoxS_promoter (E)
- 10.5ul - milli-Q watter
- 2ul - vector+SoxSpromoter
- 1.5ul - 10X EcoRI Buffer
- 1ul - EcoRI
- TOTAL = 15ul
- RBS+QseB+RBS+QseC+Term (E-P)
- 14ul - milli-Q watter
- 2ul - RBS+QseB+RBS+QseC+Term
- 2ul - 10X Buffer O
- 1ul - EcoRI
- 1ul - PstI
- TOTAL = 20ul
Tasks:
- Incubate both reactions at 37°C for 1-2 hours
- Electrophoresis to confirm
Digestions for purification:
- HlyA (E-S)
- 32ul - RBS+IL-10
- 4ul - 10X Buffer R
- 0.8ul - EcoRI
- 3.4ul - SpeI
- TOTAL = 40ul
- RBS+IL-10 (X-P)
- 32ul - RBS+IL-10
- 4ul - 10X Tango Buffer
- 1.4ul - XbaI
- 2.6ul - PstI
- TOTAL = 40ul
- RBS+IL-12 (X-P)
- 32ul - RBS+IL-12
- 4ul - 10X Tango Buffer
- 1.4ul - XbaI
- 2.6ul - PstI
- TOTAL = 40ul
- RBS+SoxR+Term (X-P)
- 32ul - RBS+SoxR+Term
- 4ul - 10X Tango Buffer
- 1.4ul - XbaI
- 2.6ul - PstI
- TOTAL = 40ul
- RBS+GFP (X-P)
- 32ul - RBS+GFP
- 4ul - 10X Tango Buffer
- 1.4ul - XbaI
- 2.6ul - PstI
- TOTAL = 40ul
- RBS+HlyB (X-P)
- 32ul - RBS+HlyB
- 4ul - 10X Tango Buffer
- 1.4ul - XbaI
- 2.6ul - PstI
- TOTAL = 40ul
- RBS+QseB+RBS+QseC+Term (X-P) (after the miniprep was checked)
- 32ul - RBS+QseB+RBS+QseC+Term
- 4ul - 10X Tango Buffer
- 1.4ul - XbaI
- 2.6ul - PstI
- TOTAL = 40ul
- vector+ConstitutivePromoter (S-P)
- 32ul - vector+Constitutive_promoter
- 4ul - 10X Tango Buffer
- 1.4ul - SpeI
- 2.6ul - PstI
- TOTAL = 40ul
- vector+FlhDC_promoter (S-P)
- 32ul - vector+FlhDC
- 4ul - 10X Tango Buffer
- 1.4ul - SpeI
- 2.6ul - PstI
- TOTAL = 40ul
- vector+SoxS_promoter (S-P) (after the miniprep was checked)
- 32ul - vector+SoxS
- 4ul - 10X Tango Buffer
- 1.4ul - SpeI
- 2.6ul - PstI
- TOTAL = 40ul
Tasks:
Incubate reactions at 37°C for 3-4hours.
Electrophoresis (loading the whole digestions volume - join two or more wells in the gel)
Purify the bands
Electrophoresis to confirm (load 5ul of gel purified digestion)
Ligation recipes
- RBS+HlyD - RBS+TolC+terminator(vector)
- 6.5ul - milli-Q water
- 10ul - RBS+HlyD (~300ng)
- 0.5ul - TolC+terminator(vector) (~150ng)
- 2ul - 10X T4 Buffer
- 1ul - T4 DNA Ligase
- TOTAL = 20ul
Tasks
Incubate all ligation reactions for 1-2 hour at 22°C.
Use 5ul of these ligations to do the transformation

 "
"