Team:TU-Delft/Project/Modelling
From 2011.igem.org








-
 Home
Home
-
 The Project
What we are doing
The Project
What we are doing
-
 The Team
Who we are
The Team
Who we are
-
 Notebooks
What we did
Notebooks
What we did
-
 Human Practice
Awareness
Human Practice
Awareness
-
 Safety
Responsibilty
Safety
Responsibilty
Modelling
Our project revolves around the addition of adhesive properties to Escherichia coli in a controlled manner. Adding the property may be regarded as the scientific part of our project, the control of the system is the engineering part. Modelling is our bridge between science and engineering. Using the scientific knowledge on our system, we can describe how the system will act and interact. Combining our mathematical model(s) with our lab results will lead to anas much realistic as possible description of our constructs and its possibilities. This description can then be used for predicting what will happen when you control the system using a specific parameter. Modelling offers the first answers to the questions posed when one engineers.
In our case, these questions are:
- How adhesive is the cell?
- How is adhesiveness dependent on induction of Mfp5?
- How do adhesive cells behave in a growth medium?
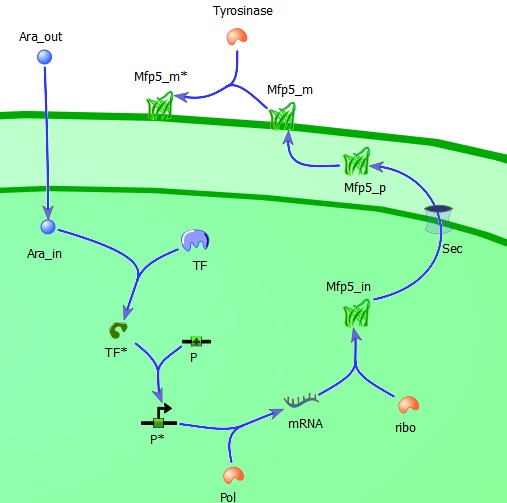
As mentioned above, to answer these questions we need to have a mechanical model. The mechanical description of the biological processes namely lays the foundation of understanding, as well as determines the framework of relations to be used in the model. We have separated the system in two parts: the intracellular (regulatory) module and the intercellular module
The intracellular module gives us information about the adhesiveness of Mfp-5 on the membrane which is directly dependent on the inducer (L-arabinose) concentration. It includes all steps in between the inducer (induction of the Mfp-5 expression?) and the amount of Mfp-5 eventually present on the outer membrane, such as for example the influence of the transcription factor, the efficiency of DNA-polymerase and the ribosome concentration in relation to the specific growth rate μ.
Extracellular L-arabinose (Ara_out) is transported inside the cell (Ara_in). Here it binds to the transcription factor (TF) which becomes active (TF*) and binds to the promotor (P). The active promotor (P*) is transcribed by DNA-polymerase (pol) and the resulting mRNA is translated by ribosomes (ribo) into intracellular Mfp5-GFP-OmpA (Mfp5_in). This is transported by the Sec transport system to the periplasm. The periplasmic Mfp5-GFP-OmpA (Mfp5_p) folds and inserts itself into the outer membrane (Mfp5_m). When tyrosinase is present tyrosine groups will be hydroxylated to L-DOPA groups resulting in adhesive membrane-bound Mfp5-GFP-OmpA (Mfp5_m*).
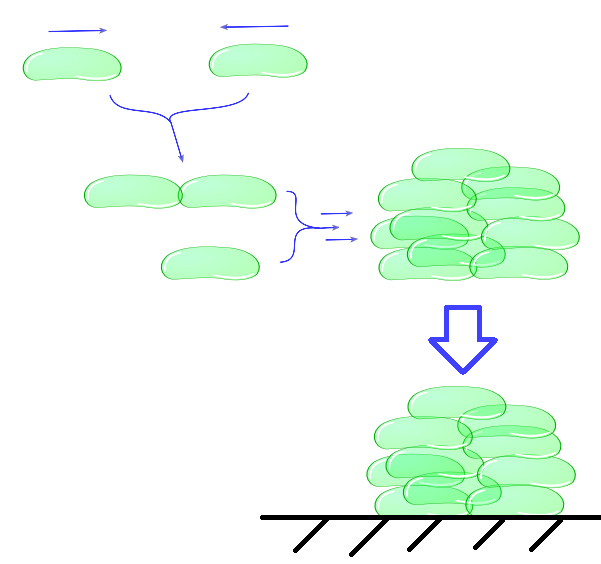
The intercellular module gives us information about cell physiology dependent on cell adhesiveness and environmental parameters. With this part of our model, we try to predict cell clustering and its related settling time. This includes factors as buoyancy, cluster formation and degradation, growth and death.(etc. not necessary because you say “includes”). In the end, our intracellular module will lead to a useful output, namely the adhesive properties of Mfp-5 in relation to the inducer. This output is directly the input for the intercellular model. Combination of as well the intracellular as the intercellular models enables us to predict the behaviour of cells in a growth medium, which is directly dependent on the concentration of the inducer L-arabinose.Individual cells bump into eachother and depending on adhesive strength of the Mfp5 and its concentration they will attach to form a larger cluster. This continuous clustering results into large heavy clusters with a high volume to surface ratio increasing their settling rate.
Retrieved from "http://2011.igem.org/Team:TU-Delft/Project/Modelling"
 "
"