Team:TU-Delft/Project/Safety/Safety-proposal
From 2011.igem.org








-
 Home
Home
-
 The Project
What we are doing
The Project
What we are doing
-
 The Team
Who we are
The Team
Who we are
-
 Notebooks
What we did
Notebooks
What we did
-
 Human Practice
Awareness
Human Practice
Awareness
-
 Safety
Responsibilty
Safety
Responsibilty
Safety proposal
If one works in a biological lab, biosafety issues deserve of great attendance. As an iGEM team, the last few months, we have to deal with biological materials almost each day. It is essential to take all the necessary safety regulations very seriously. As a result, on this page we give insight on our abundant knowledge about the safety measures which must be respected.
Biosafety Regulations
All the members of our iGEM team are students of the Technical University of Delft. Throughout the months that we are working on our project, we of course work a lot on the lab. Our lab is part of the TU Delft Faculty of Applied Sciences and, in particular, of the Kluyver laboratory for Biotechnology. Beside this, we do part of the measurements in BioNano science, at TNW. Being a biological lab, there are many biosafety rules which are essential to apply. In our case it is very important to be aware of the biological material that we are in contact with and handle every case with care, as described in the biosafety regulations.
Our faculty (Kluyver laboratory) has ML-1 laboratories and only one ML-2 laboratory. We are only allowed to work in ML-1 labs where the basic microbiology laboratory rules are applied. The rules for the use of genetically modified organisms (GMOs) are very strict. Generally, a license is required to be allowed to work with GMO’s. Only if the risks to humans and the environment are minimal to negligible, the government grants this license. The rules for working with GMO’s have to be followed by the laboratory researchers. For each laboratory one person is responsible for monitoring the appliance of these rules in that working area.
Since 1997, Dr. L.A. (Lesley) Robertson holds the position of the Biological Safety Officer (BSO) in the Faculty of Applies Sciences of Delft University of Technology. Dr. L.A. Robertson has the final responsibility for all the labs. The BSO is responsible for all the aspects of biosafety and the activities involving GMOs of the department. Her duty is to make sure that all the activities involving GMOs are done correctly and are executed carefully based on Dutch laws and regulations regarding the biosafety issues.
In order to have the permission to work in a laboratory area, we first had to pass the Biosafety Test of the BSO, which includes the presentation and identification of possible hazards in a lab. It is a test addressing all the basic rules for researchers to be allowed to work in a laboratory. This test is divided in two parts. The first one is the “hand washing test”, where we are taught to wash our hands correctly (before and after the labwork) and later on we have to be tested on how “clean of microorganisms” our hands are. It might seem to be something easy, but it is very important in order to avoid possible contaminations in the future when we are working in the lab. All team members passed this test successfully! The second part of the test is mainly based in our knowledge of all the safety regulations needed for our work which are mentioned in our “Survival Guide”.
Afterwards, to ensure that Dr. L.A. Robertson knows what happens on her laboratory, a research proposal had to be provided to her. The research proposal includes a table containing all the micro-organisms (hosts), plasmids and existing BioBricks that we intended to use. To ensure that we’ll work within the permit, there are two lists which state all the allowed vectors and hosts we can use. These lists are provided, just like the permit, by the Dutch Ministry of Housing, Spatial planning and the Environment. During the writing of our research proposal, we constantly verified in these lists if our plans were within the permit.
Only after the approval of our research proposal and when our BSO Dr. L.A. Robertson verified all the vectors and hosts, we were allowed to work on our project on the lab. Now our obligation is to inform Dr. L.A. Robertson regularly on our progression in the lab and what we are planning to do in the near future.
“Safety Issues” related to our Project
Our Parts:
Working for iGEM is a great opportunity to design your “amazing” new organism and have fun by doing that, as in every scientific project. However, there are safety issues that have to be taken seriously into account. It was very important for us, during the whole design of our iGEM project, to take into consideration all the safety parameters that our project and especially our parts could raise.
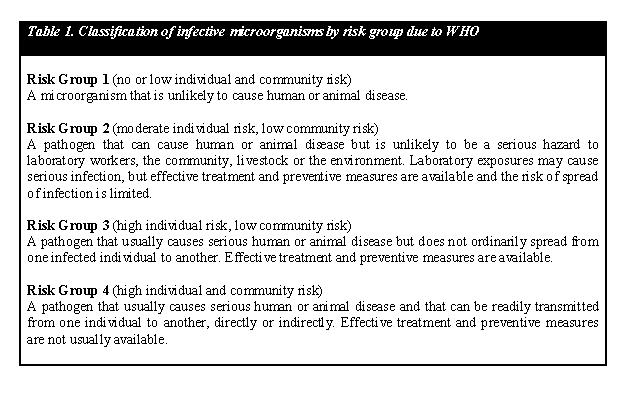
By regulation we are only permitted to work in ML-1 laboratories, and as such we are aware that we will not work with hazardous and infectious host organisms and genes. Specifically, all the genes and devices that we use should be subject to regulations as given by the Dutch Government. In our faculty of Applied Sciences and more specifically in Kluyver Laboratory of Biotechnology, our team is allowed to work only in ML-1 laboratories with organisms that are categorized into Risk Group 1, and parts which are originated from non-infectious organisms, which is explained in the table below. All of them are already commercially used systems. As a result, all the parts we will construct can be considered harmless. All our ideas and plans are verified and approved by our BSO.
Researchers’ safety:
Another safety aspect that we have to be aware of is our own safety in the laboratory, the so called “researchers’ safety”. In this case, we had to verify the safety of our biological material, but also the safety of all the techniques and chemicals that we intended to use.
As mentioned earlier, all our organisms are classified as Risk Group 1, which contains microorganisms with no recogna. While working in ML-1 laboratories, we have to deal with strains which are indicated that they have low existent virulence; however it is not non-existent virulence. These microorganisms are considered not to be hazardous for healthy persons. However, organisms labeled H1 have been shown to possibly have some infectiveness for immunocompromized persons. This seems not to be a problem in our case, because luckily all team members can be considered healthy adults.
On the other hand, regarding the laboratory techniques and chemicals that we are planning to use, there are some aspects that we have to be careful about. For our own safety in the lab we need to be careful with materials such as Bisacrylamide (cross-linking agent for the preparation of polyacrylamide gels) and ethyl bromide (chemical compound of the haloalkanes group). These materials are regarded as “dangerous, potentially carcinogenic substances”. Nevertheless, if everybody works according to a good laboratory practice, there will be no risks involved. Everybody has experience in working with possibly harmful chemicals and the proper caution is taken.
Environmental and public safety:
Last but not least, the environmental and public safety was one of our most serious considerations. In our project, the most important issue we had to consider was the choice of the host organisms that we decided to use.
The strain that we use is the E.coli strain K12, which is a specifically weakened laboratory strain. This strain is well-adapted to the laboratory environment, and unlike wild type strains, it has lost its ability to compete with natural organisms outside of the laboratory and in human organism. If it would “escape”, it would be completely unable to sustain itself without our nice little media and agar plates. Therefore our E.coli strain poses an absolute minimal threat to the public or the environment.
To conclude, because our project is basically targeted in fundamental and industrial purposes, the organisms are not designed to be released in the environment! The organisms are designed to be used in a closed system, which of course involve specific rules. But even in the case of using this organism as the solution for environmental problems, then it should be used out of the laboratory environment, it still can be used in a closed system. Due to the new properties of the organism, which can create controllable biofilm membranes, it has the advantage of its easy application just by strongly anchor to a surface. This makes our current contribution in synthetic biology lacking of environmental risk.
Biosafety for the “FUTURE”
The iGEM competition should keep working with well-known organisms. Of course projects where the toxicity of organisms can be studied and be regulated, might also be very interesting and have a huge contribution in the scientific field that we represent. However, it should be well contained and guided by experienced supervisors and scientists. Students should be well informed on lab regulations, especially when working in interdisciplinary teams. A categorized and government controlled system of lab experienced certificates should be implemented. This is already in place for nuclear laboratories, but not yet for the biohazard laboratories. This would give more clarity about who is capable to work in such environments, simultaneously creating the awareness that these hazards deserve.
An obvious solution is to confirm Event Tree Analysis (ETA) and Fault Tree Analysis (FTA) as a compulsory part of the iGEM-designing phase. This will significantly limit the risks of all iGEM projects and student will learn to take Biosafety issues into account already before they are going to work in the laboratory. Also for the Biosafety point of view and as mentioned above, in engineering organisms it is recommended to use well-known microorganism. The reason is that there is more knowledge on how internal systems work and intertwine. This will make an ETA and an FTA more effective and therefore the risks will be reduced.
The last years it is well known that there is a serious debate about the research field of synthetic biology, and generally about genetic engineering, and what this area can offer. The way that public addresses that issue is based on the personal knowledge and opinion that every one of us has of the idea of GMOs, but it also depends on the media exposure of the topic. It is a novel and “exotic” technique, something that is not naturally occurred. This fact seems to be the source of the majority of the conflicts. People believe in catastrophic consequences and in the lack of benefits that this area can offer.
Most of the times, this is the result of the limited and sometimes negative overall media coverage of the topic. The iGEM contest, representing the new generation of scientist working on the field of Genetic Engineering, should make the meaning of synthetic biology issues more clear to the public.
It is our task while working on iGEM to send out the correct view to the public about the field of synthetic biology and a very straight and easy way to do this is the proper communication between the media and scientists. As a result, it is very important that the media and the public are approached carefully and be informed properly. Negative media attention without reason should be prevented, but it should also be our duty never to cover up the truth and be aware of the consequences that we can create as scientists, as well as actively inform the public on these matters.
Retrieved from "http://2011.igem.org/Team:TU-Delft/Project/Safety/Safety-proposal"
 "
"