Team:TU-Delft/Project/Modelling
From 2011.igem.org
(Difference between revisions)
(→The Regulatory Model) |
(→The Regulatory Model) |
||
| Line 49: | Line 49: | ||
=== The Regulatory Model === | === The Regulatory Model === | ||
| - | + | [['''Description''']]: | |
| - | '''Description''' | + | |
To perform simulations, a numerical ordinary differential equation solver of MatLab was used (ode45). | To perform simulations, a numerical ordinary differential equation solver of MatLab was used (ode45). | ||
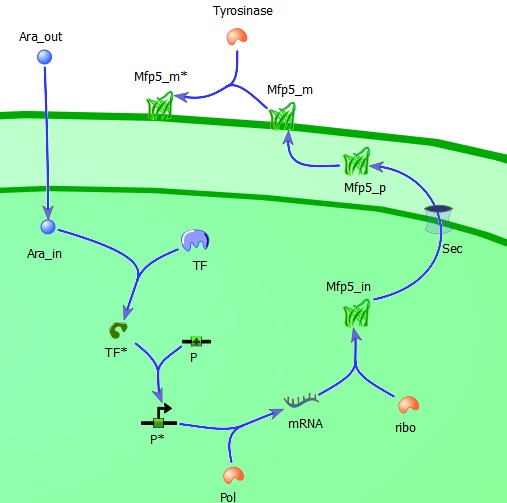
As mentioned in a previous section the model includes several assumption, mainly based on mechanisms described in literature (Alon, 2007). Following hypotheses were used to derive a mechanistic model for the production and transport of Mfp-5. <br/><br/> | As mentioned in a previous section the model includes several assumption, mainly based on mechanisms described in literature (Alon, 2007). Following hypotheses were used to derive a mechanistic model for the production and transport of Mfp-5. <br/><br/> | ||
| - | + | '''The tansport of arabinose (the inducer) across the inner cell membrane is based on diffusion kinetics.'''<br/><br/> | |
Under normal conditions, arabinose is transported into E.coli via arabinose permeases (AraE and AraFGH) (Khlebnikov et al., 2000). Because no kinetic mechanisms for the transport were available, the transport was simplified to a diffusion mechanism over the two membranes. The diffusion coefficient was based on literature; diffusion coefficient for small molecules (Alon, 2007). The assumed concentration of inducer (Carabinose_out = 55E-5 mol/L) was shown as optimal inducer concentration (Nanninger et al., 2010). The following relation was used to calculate the rate of diffusion (v_arabinose) in time (Fick’s Law): <br/><br/> | Under normal conditions, arabinose is transported into E.coli via arabinose permeases (AraE and AraFGH) (Khlebnikov et al., 2000). Because no kinetic mechanisms for the transport were available, the transport was simplified to a diffusion mechanism over the two membranes. The diffusion coefficient was based on literature; diffusion coefficient for small molecules (Alon, 2007). The assumed concentration of inducer (Carabinose_out = 55E-5 mol/L) was shown as optimal inducer concentration (Nanninger et al., 2010). The following relation was used to calculate the rate of diffusion (v_arabinose) in time (Fick’s Law): <br/><br/> | ||
| Line 64: | Line 63: | ||
In this equation, the volume of the cell equals 1 µm3 (Kubitschek et al., 1986) and the surface of the cell is calculated. Assumed is a spherical cell, whereupon Acell can be calculated by 4πR2, with R = ∛((V_cell*(3/4))/π) . <br/><br/> | In this equation, the volume of the cell equals 1 µm3 (Kubitschek et al., 1986) and the surface of the cell is calculated. Assumed is a spherical cell, whereupon Acell can be calculated by 4πR2, with R = ∛((V_cell*(3/4))/π) . <br/><br/> | ||
| - | + | '''Production of AraC and Mfp-5: protein production.'''<br/><br/> | |
When the arabinose in transported in the cell, first this will bind to the AraC-gene. This is the gene for the production of the transcription factor. After the transcription factor is produced, this will bind to the mfp-5 promoter , in order to produce the mfp-5 protein. The AraC-protein as well as the Mfp-5 protein are assumed to follow similar processes: the production of a protein. This production is divided into two steps; first transcription (copying DNA to mRNA, messenger RNA ) whereupon translation follows. Translation means the translation of mRNA to amino The following relations were assumed (Alon, 2007): <br/><br/> | When the arabinose in transported in the cell, first this will bind to the AraC-gene. This is the gene for the production of the transcription factor. After the transcription factor is produced, this will bind to the mfp-5 promoter , in order to produce the mfp-5 protein. The AraC-protein as well as the Mfp-5 protein are assumed to follow similar processes: the production of a protein. This production is divided into two steps; first transcription (copying DNA to mRNA, messenger RNA ) whereupon translation follows. Translation means the translation of mRNA to amino The following relations were assumed (Alon, 2007): <br/><br/> | ||
''Transcription : the production of mRNA'' <br/><br/> | ''Transcription : the production of mRNA'' <br/><br/> | ||
| Line 80: | Line 79: | ||
The α_(p_AraC ) is calculated by log(2)/ THalf time AraC, with THalf time ArAC = 2 min (Kolodubretz et al., 1981). | The α_(p_AraC ) is calculated by log(2)/ THalf time AraC, with THalf time ArAC = 2 min (Kolodubretz et al., 1981). | ||
The production of Mfp-5 is assumed to be similar in process, only varying the half life time of the Mfp-5 protein, which is 10 min (Haemers, 2003)<br/><br/> | The production of Mfp-5 is assumed to be similar in process, only varying the half life time of the Mfp-5 protein, which is 10 min (Haemers, 2003)<br/><br/> | ||
| - | + | '''Transport of Mfp-5''' <br/><br/> | |
Mfp5 is transported via an active mechanism. Because no kinetic mechanisms for the transport were available, the transport was simplified to a diffusion mechanism over the two membranes. The mechanism was as described in the transport of the inducer arabinose leading to the following equations (Fick’s Law):<br/> | Mfp5 is transported via an active mechanism. Because no kinetic mechanisms for the transport were available, the transport was simplified to a diffusion mechanism over the two membranes. The mechanism was as described in the transport of the inducer arabinose leading to the following equations (Fick’s Law):<br/> | ||
''Transport over inner membrane:''<br/> | ''Transport over inner membrane:''<br/> | ||
| Line 95: | Line 94: | ||
The arabinose concentration outside the cell was 55 E-5 mol/L, a relatively high concentration, which is expected to cause a high production rate of the protein. (Kleinschmidt et al. (1996)) The maximal produced mfp-5 was produced after 750s. A maximal mfp-5 concentration of 0.038 mmol/L was determined. <br/><br/> | The arabinose concentration outside the cell was 55 E-5 mol/L, a relatively high concentration, which is expected to cause a high production rate of the protein. (Kleinschmidt et al. (1996)) The maximal produced mfp-5 was produced after 750s. A maximal mfp-5 concentration of 0.038 mmol/L was determined. <br/><br/> | ||
| - | + | [['''The Matlab Code''']]<br/><br/> | |
Revision as of 20:44, 30 January 2012








 "
"