Team:Cambridge/Project/In Vitro
From 2011.igem.org
Contents |
Isolating Reflectin and Making Thin Films
Previous in vitro investigations of reflectin showed that it was possible to use a method called flow coating to deposit a thin layer of reflectin onto a silicon substrate which would then demonstrate structural colour.
We wanted to investigate using a different coating method – spin coating – and to try out different methods for protein purification. A polyhis-tagged variant of the protein was overexpressed and purified using a polyhis affinity column. Thin films were made by precipitating with either acetone or ethanol and resuspending in HFIP before either spin coating or flow coating onto a silicon wafer.
We also investigated an inclusion body prep, as the protein we found that the protein formed inclusion bodies upon over-expression.
By varying our purification and coating methods, we were able to create vibrant thin films.
Polyhis-Tagging Reflectin
The reflectin genes were polyhis-tagged by incorporating the sequence of part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K128005 BBa_K128005] into the primers used to clone the gene. Polyhis tagging was chosen because it allows proteins to be purified with relatively simple apparatus, an affinity column, to which a metal containing resin is added traps the protein when cell lysate is added.
Over-Expression
Reflectin was placed on a high copy plasmid ([http://partsregistry.org/Part:pSB1A2 PSB1A2]) under an arabinose inducible promoter ([http://partsregistry.org/Part:BBa_I0500 pBad]) in order to express reflectin at a high level. As well as a negative control, we used an sfGFP-reflectin fusion as a control for reflecitn production.
In the non-GFP cells we found that there were brightly lit points at the ends of nearly every cell, which were not present in the negative control. In the GFP fusion cells, these spots glowed strongly, while the rest of the cell was relatively dark. This indicated that reflectin produces inclusion bodies when expressed at high level.
We really need the images here! - at least, non GFP reflectin inclusion bodies & GFP inclusion bodies. would be nice to have the negative control as well. -- Haydn
Extraction & Purification
We investigated two principal methods for extracting the protein from the transformed E. coli - we lysed the cells and ran the lysate through a polyhis affinity column and we tried a proprietary inclusion body prep.
Polyhis-Tag Affinity Column
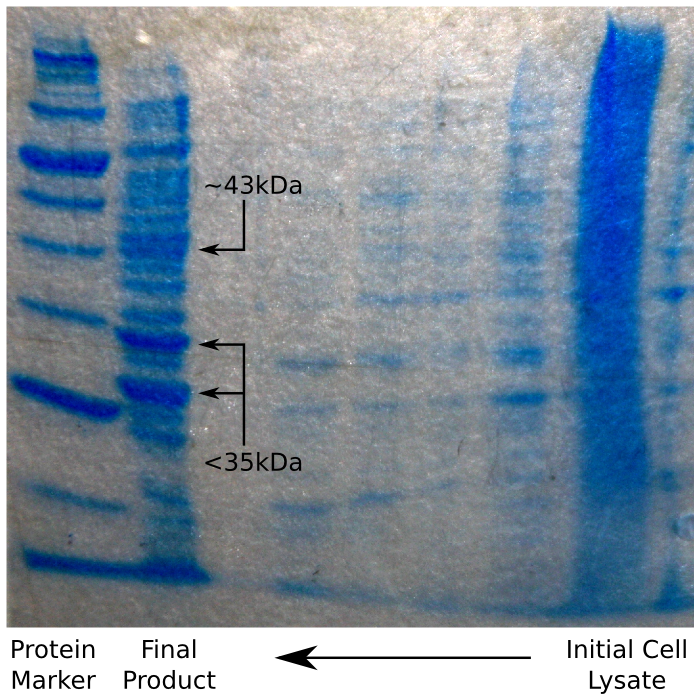
The polyhis affinity column allowed us to extract our tagged protein from the lysate without too much difficulty. In order to increase the purity of our sample we tried using an acetone or ethanol precipitation step to remove chemicals retained in the elution buffer and to concentrate the protein for downstream processing.
Having done this, we ran our sample on an SDS Page protein gel, to verify that we had in fact purified reflectin. We were expecting to see a thick band of reflectin at around 35 kDa, which – as the image shows – was the case.
Inclusion Body Prep
The Norgen Proteospin inclusion body prep kit was experimented with as a commercial alternative to the previous purification method. The kit was simple to use, but required the use of an ultracentrifuge capable of spinning up to 25ml of fluid at 27,000g. It was found that the spinning forces specified in the protocol for these steps weren't capable of moving fluid through the necessary columns and higher forces were needed.
On analysing the end-product of the purification with SDS-PAGE approximately 20 discrete bands were observed from 10-200 kda with 2 bold bands, one of which corresponded to the size of reflectin.
As the gel shows, the resulting protein is not as pure as the result of the previous method as the inclusion bodies do not contain only reflectin. However, greater purity might have been achieved by using the result of the inclusion body prep as the first stage of the polyhis affinity protocol, as it would reduce the amount of non-polyhis protein going in to the column. This was not tested in practice, however, as our protein appeared to be pure enough for processing, and we did not have the time to experiment further.
Thin Films
In order to demonstrate structural colour, thin films were created by re-suspending the purified protein in HFIP and spin coating or flow coating the re-suspended protein onto a silicon substrate. This gave us brightly coloured thin films which responded to water vapour in the air by changing colour. We performed controls by making HFIP films and films with Bovine Serum Albumin (BSA, a generic protein) neither of which showed any structural colour.
Our initial films were not uniform in colour and would form crystals when allowed to dry out. We improved this by increasing the purity of our protein as well as refining our coating technique.
Future Work
We wanted to investigate how to make more uniform films as well as improving the intensity of the colour and reducing the crystallisation. We also wanted to investigate whether we could control the colour of the films electrically.
 "
"