Team:Cambridge/Project
From 2011.igem.org
(→Project breakdown) |
|||
| Line 5: | Line 5: | ||
''Bact'''iridescence''''' is a project based around the unique properties of reflectin, a squid protein with the highest refractive index of any known proteinaceous substance. In squid this protein forms complex platelets which act as [http://en.wikipedia.org/wiki/Distributed_Bragg_reflector Bragg reflectors] to provide camouflage. We aim to express reflectin in E. coli and optimise the optical properties in order to build the groundwork for the manipulation of living structural colour. [[Team:Cambridge/Project/Background| Read more about reflectin and structural colour here.]] | ''Bact'''iridescence''''' is a project based around the unique properties of reflectin, a squid protein with the highest refractive index of any known proteinaceous substance. In squid this protein forms complex platelets which act as [http://en.wikipedia.org/wiki/Distributed_Bragg_reflector Bragg reflectors] to provide camouflage. We aim to express reflectin in E. coli and optimise the optical properties in order to build the groundwork for the manipulation of living structural colour. [[Team:Cambridge/Project/Background| Read more about reflectin and structural colour here.]] | ||
| - | =Project breakdown= | + | ==Project breakdown== |
| - | We broke down our project into clear stages, with highly focussed objectives for each. Here, we outline | + | We broke down our project into clear stages, with highly focussed objectives for each. Here, we outline how our plans were designed and modified in order to achieve each objective to as high a standard as possible. Our [experiments] are also put into context via links in this section. |
| + | |||
| + | *[Preliminary observations] | ||
| + | *[Objective One] - expression of recombinant reflectin in E. coli | ||
=Preliminary observations= | =Preliminary observations= | ||
Revision as of 14:51, 10 August 2011
Contents |
Project breakdown
We broke down our project into clear stages, with highly focussed objectives for each. Here, we outline how our plans were designed and modified in order to achieve each objective to as high a standard as possible. Our [experiments] are also put into context via links in this section.
- [Preliminary observations]
- [Objective One] - expression of recombinant reflectin in E. coli
Preliminary observations
In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin in vivo. We therefore obtained several specimens of loligo opalescens and loligo vulgaris squid from a local seafood restaurant and an online fishing bait store for dissection. We chose these species because the whole family of loliginid squid has been identified to contain reflectin, and these particular species were the only members of the family available to us. We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following protocol. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli, and definitely enthused the team to obtain bactiridescence!
Objective One - Express reflectin in E. coli
Our first objective was to try to express reflectin, in any shape or form, in E. coli. Ideally, we'd be able to express reflectin in the bacteria at a range of levels using a single construct, so we can both overexpress (for in vitro studies) or underexpress (to promote in vivo folding) straightforwardly. In order to do this, we had to create an expression plasmid for reflectin with which we could transform the bacterial cells. Hence, we had to isolate a reflectin gene sequence, choose a suitable promotor and join them on an appropriate backbone in order to engineer what we wanted. These steps are outlined below.
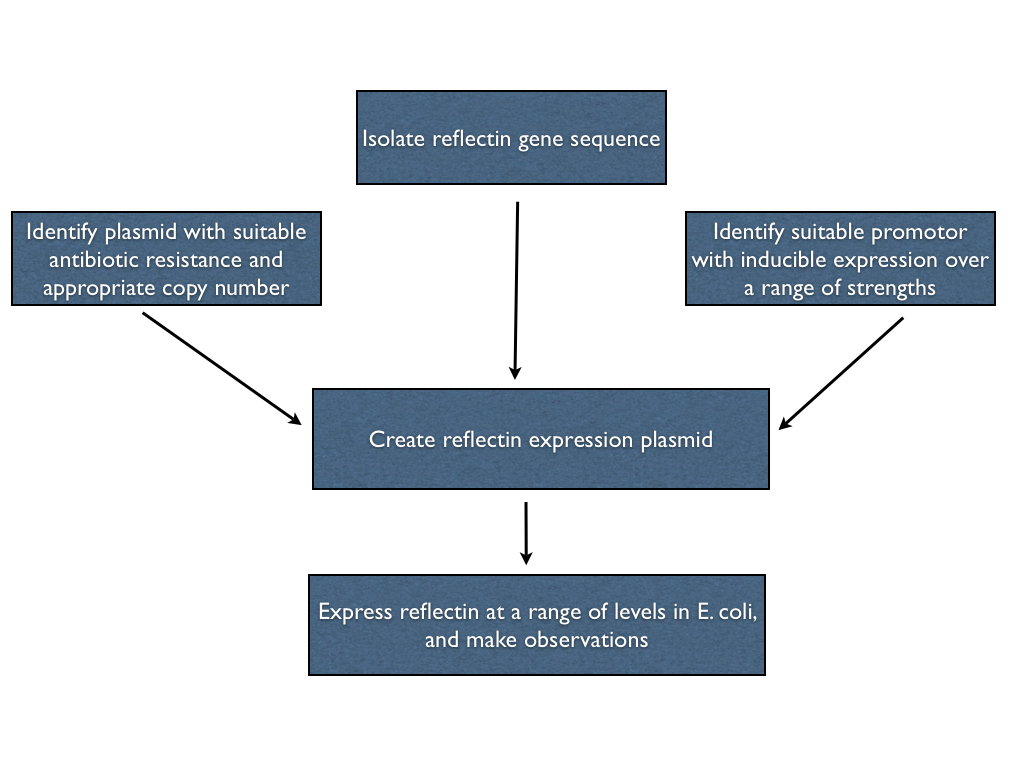
Isolating the reflectin gene
This was more complicated than we initially expected. Our first idea was to attempt to clone reflectin genes from genomic DNA, extracted from the same squid specimens that we dissected for our [preliminary observations]. We started with a very crude genomic extraction protocol, but unfortunately we did not manage to successfully clone reflectin using this method (details of the experiment can be found [here]). We then attempted two cleaner genomic extraction protocols (described [here]), but unfortunately we remained unsuccessful. After some discussion with Dr. Wendy Goodson, a researcher who was one of the first to study reflectin, we found out that cloning from cephalopod genomic DNA is notoriously difficult, although the cause for this is not understood. She then very kindly offered to send us a quantity of cloning plasmids containing reflectin genes that she has worked with in the past; in this way we obtained genes for reflectin A1, A2 and 1B that had already been codon optimised for expression in E. coli. The amplification of these plasmids is described [here].
Selecting the promotor
Selecting the backbone
Creating the expression plasmid
Hopefully coming soon!
Expression in E. coli
Hopefully coming soon!
References
[http://www.nature.com/nmat/journal/v6/n7/abs/nmat1930.html] Kramer et al. The self-organizing properties of squid reflectin protein Nature Materials 533-538 VOL6 JULY 2007 [http://www.sciencemag.org/content/303/5655/235.short] Crookes et al. Reflectins: The Unusual Proteins of Squid Reflective Tissues SCIENCE 235-238 VOL303 9 JANUARY 2004 [http://www.sciencedirect.com/science/article/pii/S0142961209011442] Morse et al. The role of protein assembly in dynamically tunable bio-optical tissues Biomaterials 793-801 VOL31 FEBRUARY 2010 [http://www.publish.csiro.au/paper/ZO9920319.html] Griffiths et al. Iridophores in the mantle of giant clams. Australian Journal of Zoology (1992) Volume: 40, Issue: 3 Pages: 319-326 [http://www.ncbi.nlm.nih.gov/pubmed/19776150] Izumi et al. Changes in reflectin protein phosphorylation are associated with dynamic iridescence in squid. J. R. Soc. Interface 6 March 2010 vol. 7 no. 44 549-560 [http://www.springerlink.com/content/bba14b73ad35f495/]Brocco et al. Reflector cells in the skin of Octopus dofleini Cell and Tissue Research, Volume 205, Number 2, 167-186, 1980
 "
"
