Team:Cambridge/Experiments/Protein Purification
From 2011.igem.org
(→His-Trap Protein Purification - First Attempt) |
|||
| Line 1: | Line 1: | ||
{{Template:Team:Cambridge/CAM_2011_TEMPLATE_HEAD}} | {{Template:Team:Cambridge/CAM_2011_TEMPLATE_HEAD}} | ||
| - | =His-Trap Protein Purification - First Attempt= | + | ===His-Trap Protein Purification - First Attempt=== |
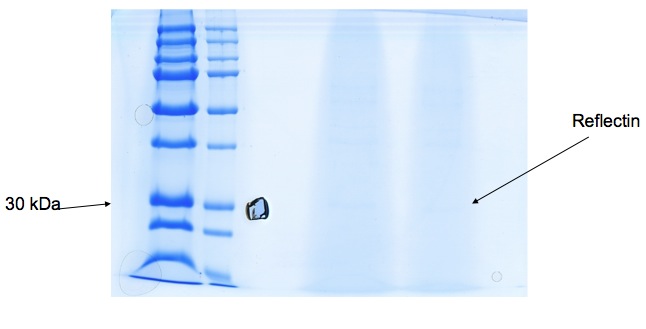
Bacteria expressing his-tagged reflectin were lysed, and the protein was purified using a his-trap column and a denaturing protocol in order to solubilise reflectin. Polyacrylamide gel electrophoresis suggests we have a protein around 30kDa in weight which correlates with the molecular weight of reflectin, but a lack of other evidence means reflectin's presence is not yet certain. | Bacteria expressing his-tagged reflectin were lysed, and the protein was purified using a his-trap column and a denaturing protocol in order to solubilise reflectin. Polyacrylamide gel electrophoresis suggests we have a protein around 30kDa in weight which correlates with the molecular weight of reflectin, but a lack of other evidence means reflectin's presence is not yet certain. | ||
| - | ==Practice== | + | ====Practice==== |
*After high copy expression plasmids for his-tagged reflectin were successfully [https://2011.igem.org/Team:Cambridge/Experiments/Assembly_of_Reflectin_Constructs assembled] and [https://2011.igem.org/Team:Cambridge/Protocols/Transformation_of_E.Coli transformed] in E. coli, [https://2011.igem.org/Team:Cambridge/Protocols/Overnight_Culture cell cultures] were incubated overnight with 1mM arabinose to induce reflectin expression. | *After high copy expression plasmids for his-tagged reflectin were successfully [https://2011.igem.org/Team:Cambridge/Experiments/Assembly_of_Reflectin_Constructs assembled] and [https://2011.igem.org/Team:Cambridge/Protocols/Transformation_of_E.Coli transformed] in E. coli, [https://2011.igem.org/Team:Cambridge/Protocols/Overnight_Culture cell cultures] were incubated overnight with 1mM arabinose to induce reflectin expression. | ||
*[https://2011.igem.org/Team:Cambridge/Protocols/Buffers Buffers] were prepared, and their pH checked and readjusted if necessary on the day of purification. | *[https://2011.igem.org/Team:Cambridge/Protocols/Buffers Buffers] were prepared, and their pH checked and readjusted if necessary on the day of purification. | ||
| Line 10: | Line 10: | ||
*This procedure was performed using two different cultures of the same bacteria. The culture that produced the highest yield of reflectin was selected as a 'seed' for future cultures that would be used to harvest more protein. | *This procedure was performed using two different cultures of the same bacteria. The culture that produced the highest yield of reflectin was selected as a 'seed' for future cultures that would be used to harvest more protein. | ||
| - | ==Results== | + | ====Results==== |
Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 0.4mg of reflectin from the column for the denser culture, and 0.2mg from the other. After dialysis, a vacuum centrifuge was used to precipitate the protein. this precipitate was taken to the Nanophotonics centre in order to make our first reflectin thin films, and SDS-PAGE was run by another member of the department to check whether or not reflectin was contained in the precipitate. | Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 0.4mg of reflectin from the column for the denser culture, and 0.2mg from the other. After dialysis, a vacuum centrifuge was used to precipitate the protein. this precipitate was taken to the Nanophotonics centre in order to make our first reflectin thin films, and SDS-PAGE was run by another member of the department to check whether or not reflectin was contained in the precipitate. | ||
| Line 20: | Line 20: | ||
Since this was the first time any of us had tried to perform the his-trap technique, we also learnt some valuable lessons from our experiences in this first experiment. Some helpful 'tips' have been added to the [https://2011.igem.org/Team:Cambridge/Protocols/Protein_Purification his-trap purification protocol] as a result, which really helped us to improve our yield of reflectin using this method. | Since this was the first time any of us had tried to perform the his-trap technique, we also learnt some valuable lessons from our experiences in this first experiment. Some helpful 'tips' have been added to the [https://2011.igem.org/Team:Cambridge/Protocols/Protein_Purification his-trap purification protocol] as a result, which really helped us to improve our yield of reflectin using this method. | ||
| + | |||
| + | |||
| + | ===His-Trap Protein Purification - Second Attempt=== | ||
| + | Reflectin was harvested from bacteria very similarly to the [https://2011.igem.org/Team:Cambridge/Experiments/Protein_Purification first attempt], this time attempting both variants of the [https://2011.igem.org/Team:Cambridge/Protocols/Protein_Purification purification protocol] and both nickel and ABT cobalt resin (kindly donated by Carole Augustus, Thistle Scientific). The culture that provided the higher yield of reflectin last time was harvested in this attempt. | ||
| + | |||
| + | ====Practice==== | ||
| + | The methods described in the first attempt at his-trap purification were repeated with two new columns, one containing nickel resin and the other [[Team:Cambridge/Protocols/Protein_Purification | tips]] we developed last time, while in the cobalt column the [variant] was used. The cobalt was also washed through with 6 extra column volumes of sterile water and triple the binding buffer at the start of the protocol, as recommended by the manufacturer. | ||
| + | Instead of protein precipitation with dialysis, we attempted [acetone precipitation] and [ethanol precipitation] to fully isolate the reflectin product. | ||
| + | |||
| + | ====Results==== | ||
| + | Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 2mg of reflectin from each column, indicating a five-fold increase in our yield. We therefore favour the 'variant' his-trap protocol, since it is slightly easier to perform, and seems to be more robust (it is harder to go wrong!). | ||
| + | Both acetone and ethanol precipitation worked well, with large pellets visible at the end of the procedures. | ||
{{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | {{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | ||
Revision as of 09:15, 16 September 2011
Contents |
His-Trap Protein Purification - First Attempt
Bacteria expressing his-tagged reflectin were lysed, and the protein was purified using a his-trap column and a denaturing protocol in order to solubilise reflectin. Polyacrylamide gel electrophoresis suggests we have a protein around 30kDa in weight which correlates with the molecular weight of reflectin, but a lack of other evidence means reflectin's presence is not yet certain.
Practice
- After high copy expression plasmids for his-tagged reflectin were successfully assembled and transformed in E. coli, cell cultures were incubated overnight with 1mM arabinose to induce reflectin expression.
- Buffers were prepared, and their pH checked and readjusted if necessary on the day of purification.
- An inclusion body prep was performed with 50ml of overnight culture. Reflectin was purified from the resulting lysate using a his-trap column.
- This procedure was performed using two different cultures of the same bacteria. The culture that produced the highest yield of reflectin was selected as a 'seed' for future cultures that would be used to harvest more protein.
Results
Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 0.4mg of reflectin from the column for the denser culture, and 0.2mg from the other. After dialysis, a vacuum centrifuge was used to precipitate the protein. this precipitate was taken to the Nanophotonics centre in order to make our first reflectin thin films, and SDS-PAGE was run by another member of the department to check whether or not reflectin was contained in the precipitate.
We received gel image (see below) back from Kathryn Lilley who kindly offered to run a protein gel for us. It's not initally clear (try tilting your laptop screen back!), but a band at 30-36kDa suggests Reflectin may be present. We're hoping to use mass spectrometry to confirm this tentative result.
Despite not being certain we had reflectin, we pressed on with our thin films experiment, which produced some very interesting results!
Since this was the first time any of us had tried to perform the his-trap technique, we also learnt some valuable lessons from our experiences in this first experiment. Some helpful 'tips' have been added to the his-trap purification protocol as a result, which really helped us to improve our yield of reflectin using this method.
His-Trap Protein Purification - Second Attempt
Reflectin was harvested from bacteria very similarly to the first attempt, this time attempting both variants of the purification protocol and both nickel and ABT cobalt resin (kindly donated by Carole Augustus, Thistle Scientific). The culture that provided the higher yield of reflectin last time was harvested in this attempt.
Practice
The methods described in the first attempt at his-trap purification were repeated with two new columns, one containing nickel resin and the other tips we developed last time, while in the cobalt column the [variant] was used. The cobalt was also washed through with 6 extra column volumes of sterile water and triple the binding buffer at the start of the protocol, as recommended by the manufacturer. Instead of protein precipitation with dialysis, we attempted [acetone precipitation] and [ethanol precipitation] to fully isolate the reflectin product.
Results
Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 2mg of reflectin from each column, indicating a five-fold increase in our yield. We therefore favour the 'variant' his-trap protocol, since it is slightly easier to perform, and seems to be more robust (it is harder to go wrong!). Both acetone and ethanol precipitation worked well, with large pellets visible at the end of the procedures.
 "
"