Team:Cambridge/Project/Microscopy
From 2011.igem.org
(→Reflectin Thin Films) |
|||
| (14 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Team:Cambridge/CAM_2011_TEMPLATE_HEAD}} | {{Template:Team:Cambridge/CAM_2011_TEMPLATE_HEAD}} | ||
| - | + | =Microscopy= | |
| + | |||
| + | This page collects our observations made on a confocal microscope. Thanks go to Paul Grant and Fernan Federici for their help in obtaining these images. | ||
| + | |||
==Preliminary observations== | ==Preliminary observations== | ||
In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin ''in vivo''. We therefore obtained several specimens of ''Loligo opalescens'' and ''Loligo vulgaris'' squid from a local seafood restaurant and an online fishing bait store for dissection. We chose these species because the whole family of loliginid squid has been identified to contain reflectin, and these particular species were the only members of the family available to us. | In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin ''in vivo''. We therefore obtained several specimens of ''Loligo opalescens'' and ''Loligo vulgaris'' squid from a local seafood restaurant and an online fishing bait store for dissection. We chose these species because the whole family of loliginid squid has been identified to contain reflectin, and these particular species were the only members of the family available to us. | ||
| - | We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following [https://2011.igem.org/Team:Cambridge/Protocols/Confocal_Microscopy_of_Loligo_Eye_and_Mantle_Dermis_Samples protocol]. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli | + | |
| - | + | We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following [https://2011.igem.org/Team:Cambridge/Protocols/Confocal_Microscopy_of_Loligo_Eye_and_Mantle_Dermis_Samples protocol]. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli. | |
| - | =Squid Tissues= | + | |
| + | ===Squid Tissues=== | ||
[[File:Squideye reflectin250repeat.gif| 300px | thumb | left | This animation is composed of layers taken as the microscope was focused through the layers of a sample of reflective squid cells from the eye cup mounted in Phosphate buffered saline]] | [[File:Squideye reflectin250repeat.gif| 300px | thumb | left | This animation is composed of layers taken as the microscope was focused through the layers of a sample of reflective squid cells from the eye cup mounted in Phosphate buffered saline]] | ||
| Line 13: | Line 17: | ||
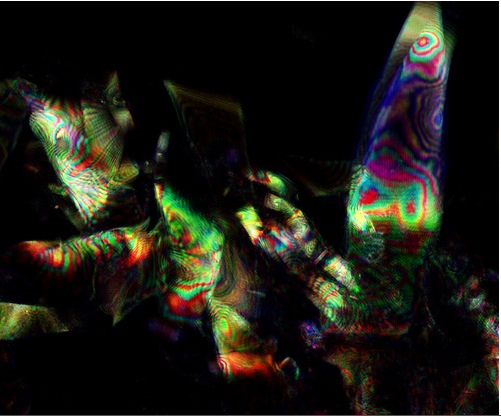
[[File:Iridescent cells from around squid eye.jpg |thumb| 600px| center| Iridescent cells from around the squid eye, with thanks to Fernan Federici and Paul Grant]] | [[File:Iridescent cells from around squid eye.jpg |thumb| 600px| center| Iridescent cells from around the squid eye, with thanks to Fernan Federici and Paul Grant]] | ||
| - | We used the enzyme trypsin to isolate cells from the mantle of the squid as this frees them from the extracellular matrix. Again we used the confocal microscope in a configuration that detects reflected light. | + | We used the enzyme trypsin to isolate cells from the mantle of the squid as this frees them from the extracellular matrix. Again we used the confocal microscope in a configuration that detects reflected light. Below is a non-iridescent cell from the tentacle imaged under the same conditions. |
| - | + | ||
| - | =Reflectin Expressing Cells= | + | <center> |
| + | <gallery widths='300px' heights='300px' caption='An iridescent squid cell, alongside a non-iridescent cell using the same confocal settings. Thanks to [[Team:Cambridge/Team/Academics#Paul_Grant | Paul Grant]] for his help imaging these.'> | ||
| + | File:Trypsinogised Mantle Tissue_1.jpg | Iridescent spindle shaped cell from the squid mantle, isolated using trypsin | ||
| + | File:Cam Squidtentaclenegativecontrol.jpg | Non-iridescent control cell from the squid tentacle | ||
| + | </gallery> | ||
| + | </center> | ||
| + | |||
| + | ===Reflectin Expressing Cells=== | ||
| - | These cells are producing a reflectin A1-GFP fusion protein | + | These cells are producing a reflectin A1-GFP fusion protein from a high-copy plasmid (pSB1A3) showing fluorescent inclusion bodies. |
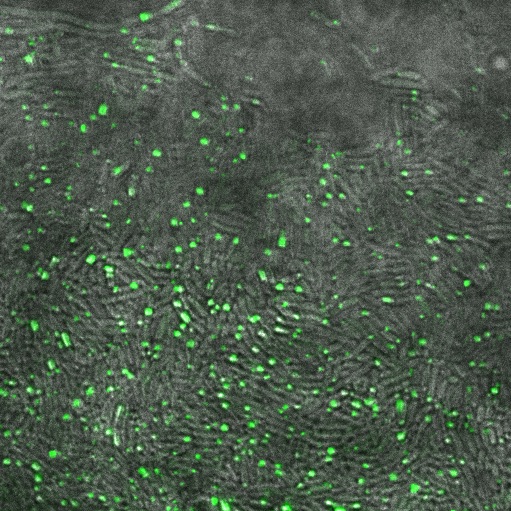
[[File:Cam Reflectin-GFP-inclusionbodies.jpg | thumb| 600px| center| ''E. coli'' transformed with our pBAD-ReflectinA1-GFP construct, induced by adding 1mM arabinose]] | [[File:Cam Reflectin-GFP-inclusionbodies.jpg | thumb| 600px| center| ''E. coli'' transformed with our pBAD-ReflectinA1-GFP construct, induced by adding 1mM arabinose]] | ||
| Line 28: | Line 38: | ||
The following confocal micrographs show control bacteria, transformed but uninduced bacteria and bacteria containing our construct and induced to produce reflectin A1. Interestingly, all cells display some reflectance, and perhaps surprsingly the induced cells show the least overall reflectanct. The induced cells display punctate dots of reflectance, which suggests inclusion bodies have been formed. However, despite uniform imaging settings across the data set, there is a differing amount of reflectance from the medium the cells are growing on. Further work we would like to carry out would involve producing slides of the same bacteria but using gelatin and PBS to minimise the background reflectance, compare reflectin and non-reflectin inclusion bodies under various imaging techniques and make use of a spectrometer to gather spectral data on the reflectin expressing bacteria, as the limitations of reflected light confocal microscopy - namely background reflectance, is highlighted by these images. | The following confocal micrographs show control bacteria, transformed but uninduced bacteria and bacteria containing our construct and induced to produce reflectin A1. Interestingly, all cells display some reflectance, and perhaps surprsingly the induced cells show the least overall reflectanct. The induced cells display punctate dots of reflectance, which suggests inclusion bodies have been formed. However, despite uniform imaging settings across the data set, there is a differing amount of reflectance from the medium the cells are growing on. Further work we would like to carry out would involve producing slides of the same bacteria but using gelatin and PBS to minimise the background reflectance, compare reflectin and non-reflectin inclusion bodies under various imaging techniques and make use of a spectrometer to gather spectral data on the reflectin expressing bacteria, as the limitations of reflected light confocal microscopy - namely background reflectance, is highlighted by these images. | ||
| - | + | <center> | |
| - | + | <gallery> | |
| - | + | File:Cam PBAD-His-Reflectin-negativecontrol1.jpg | ''E. coli'' negative control | |
| + | File:Cam PBAD-His-Reflectin-uninduced1.jpg | ''E. coli'' transformed with our pBAD-His-ReflectinA1 construct, but not induced - no addition of arabinose | ||
| + | File:Cam PBAD-His-Reflectin-induced.jpg | ''E. coli'' transformed with our pBAD-His-ReflectinA1 construct, induced by adding 1mM arabinose | ||
| + | </gallery> | ||
| + | </center> | ||
| - | =Reflectin Thin Films= | + | ===Reflectin Thin Films=== |
| + | |||
| + | Below is a selection of our thin film micrographs, including the relevant controls. | ||
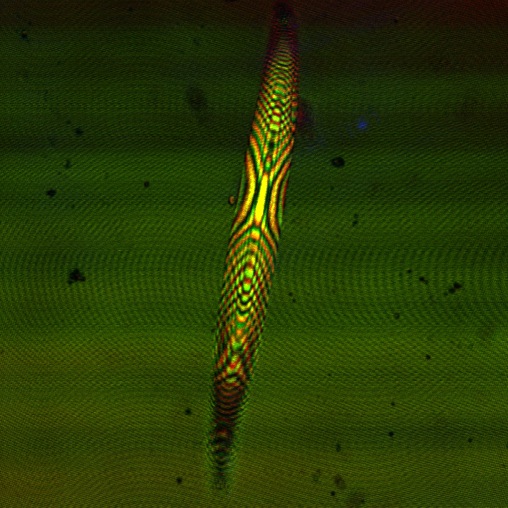
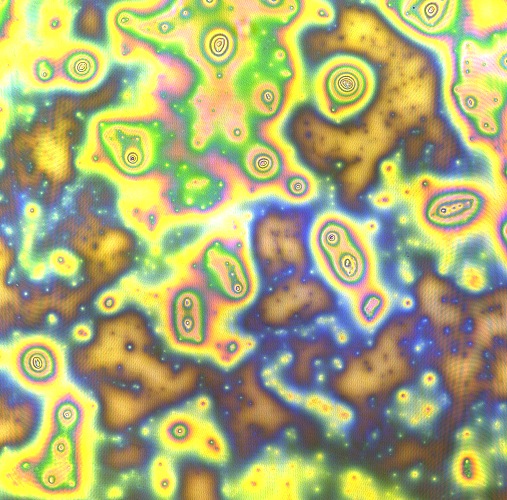
[[File:Cam Reflectin thin film confocal.jpg |thumb | 600px | center | A Confocal Micrograph of one of our first reflectin thin films, showing a great deal of impurities and non-uniformity. ]] | [[File:Cam Reflectin thin film confocal.jpg |thumb | 600px | center | A Confocal Micrograph of one of our first reflectin thin films, showing a great deal of impurities and non-uniformity. ]] | ||
| + | <center> | ||
| + | <gallery widths='250px'> | ||
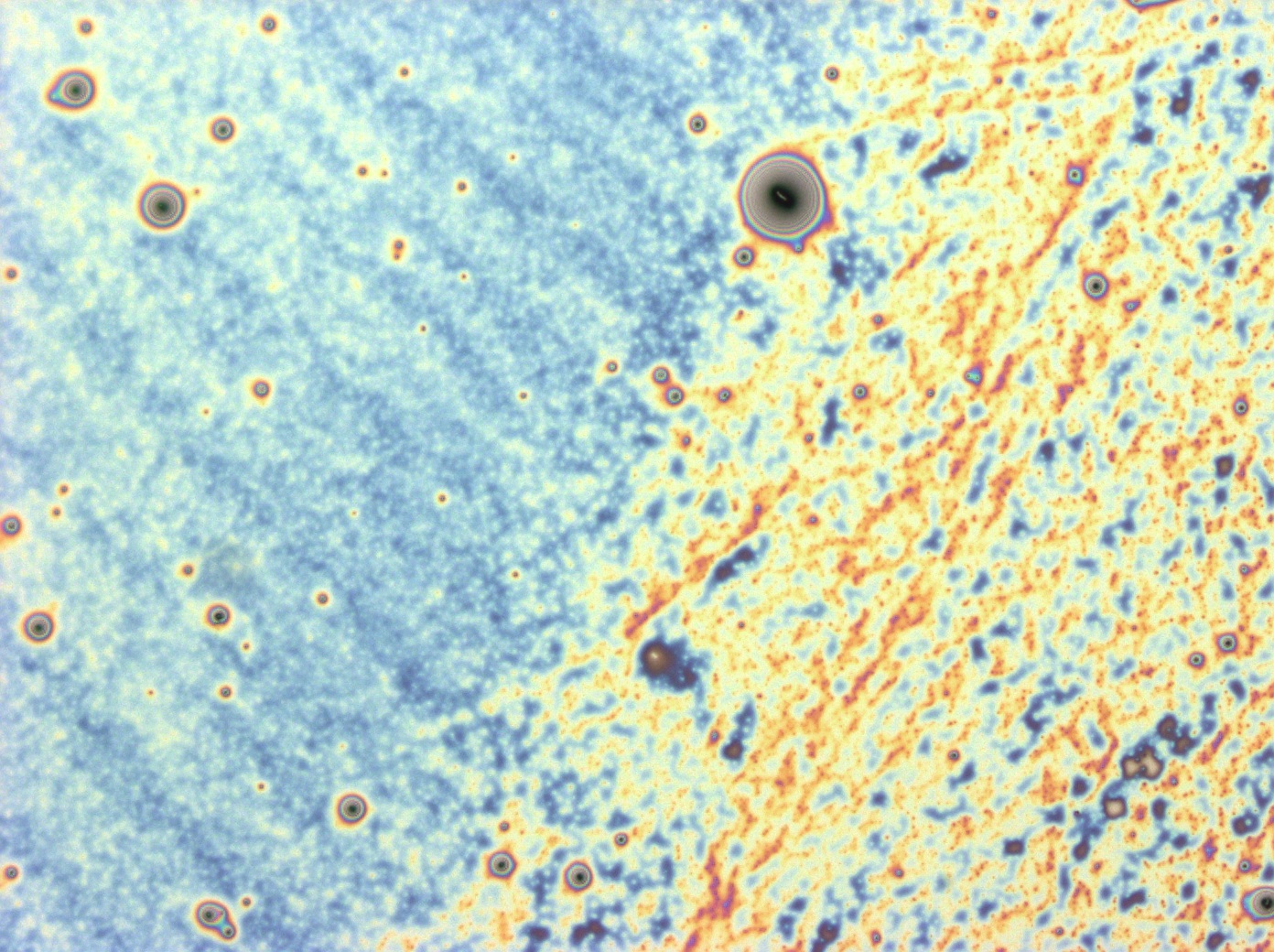
| + | File:Cam Reflectin Thin Film2.jpg | A light micrograph of a thin film made with centrifuged reflectin HFIP solution. A change in colour from blue to yellow can be seen, suggesting only one severe non-uniformity in the height of the film | ||
| + | File:Cam ReflectinFilm Centrifuged.jpg | A light micrograph of a thin film made with centrifuged reflectin HFIP solution. The centrifugation step removed a lot of the impurited and this film exhibits a much more uniform colouration. | ||
| + | </gallery> | ||
| + | </center> | ||
| - | + | <center> | |
| - | + | <gallery widths='200px' caption='Two of our thin film controls.'> | |
| - | + | ||
| - | + | ||
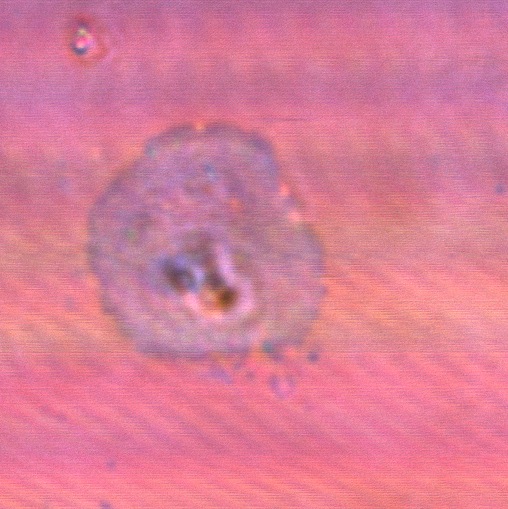
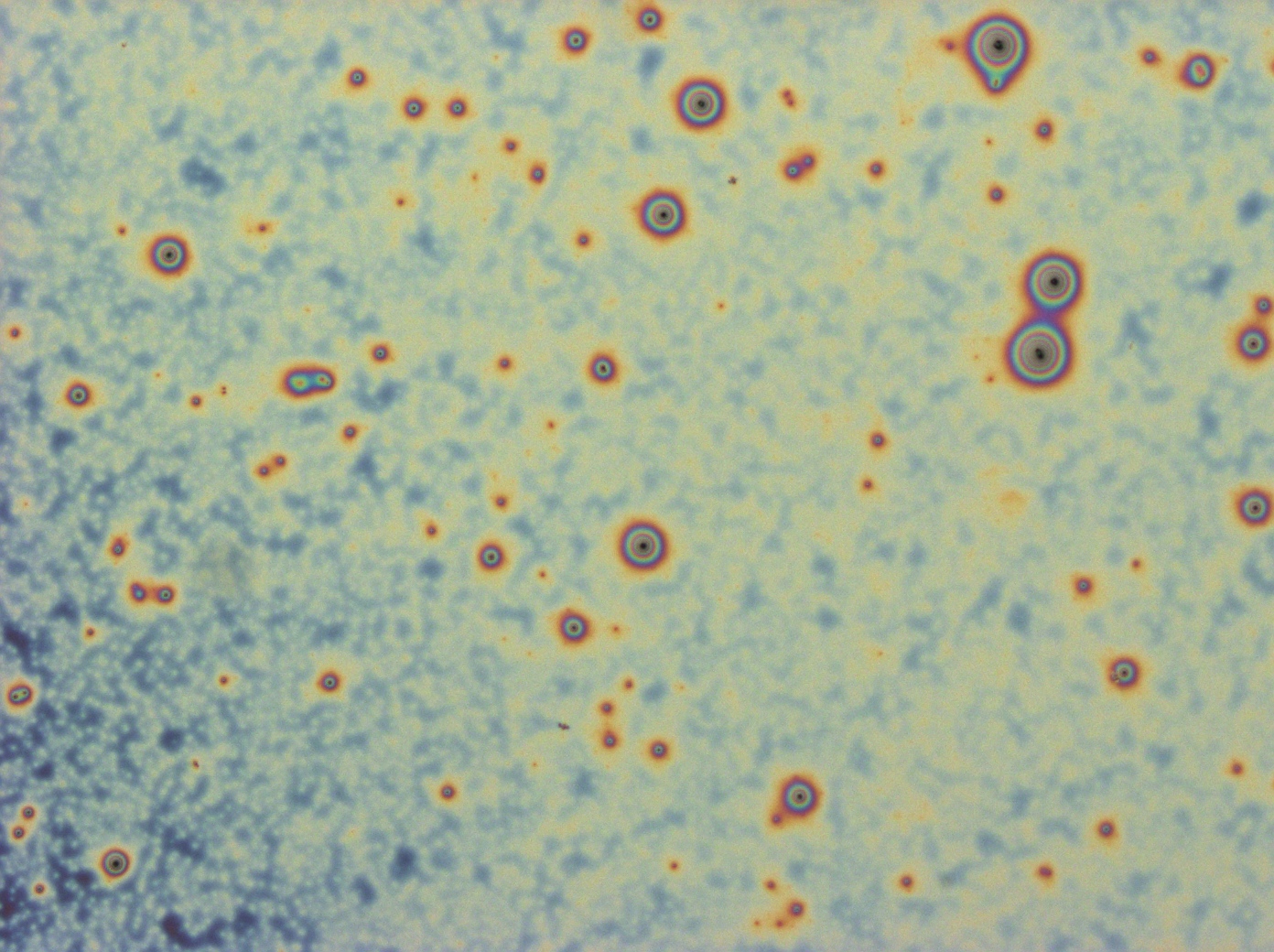
File:Cam HFIP only control thinfilm.jpg|''HFIP (solvent) only control does not exhibit iridescence'' | File:Cam HFIP only control thinfilm.jpg|''HFIP (solvent) only control does not exhibit iridescence'' | ||
File:BSAcontrolfilm1.jpg| ''Bovine Serum Albumin makes a dull, striated thin film'' | File:BSAcontrolfilm1.jpg| ''Bovine Serum Albumin makes a dull, striated thin film'' | ||
</gallery> | </gallery> | ||
| + | </center> | ||
| - | + | <center> | |
| - | + | <gallery widths='300px' heights='300px' perrow=2 caption='Some examples of multilayer films'> | |
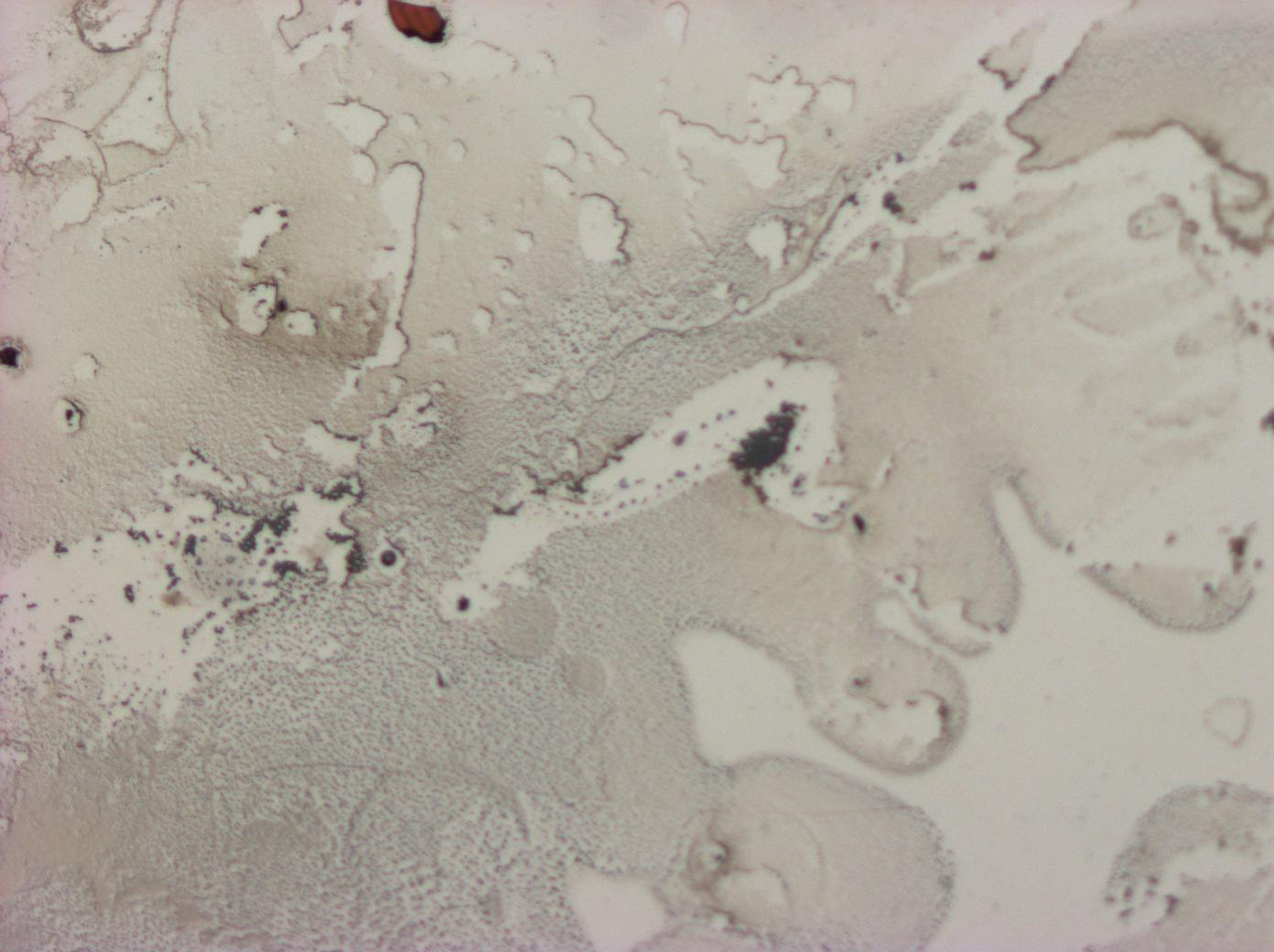
| - | + | File:Cam Multilayer drop 1.jpg | A light micrograph of a reflectin thin film multilayer | |
| - | + | File:Cam Crazy multilayer single AP 2k spin2nd.jpg | A light micrograph of a PDMS on reflectin thin film - PDMS multilayer | |
| - | + | File:Cam crazy multilayer2.jpg | Another light micrograph of a PDMS on reflectin thin film multilayer | |
| - | + | File:Cam crazy multilayer3.jpg | A PDMS on reflectin multilayer thin film | |
| + | </gallery> | ||
| + | </center> | ||
{{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | {{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | ||
Latest revision as of 22:37, 21 September 2011
Contents |
Microscopy
This page collects our observations made on a confocal microscope. Thanks go to Paul Grant and Fernan Federici for their help in obtaining these images.
Preliminary observations
In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin in vivo. We therefore obtained several specimens of Loligo opalescens and Loligo vulgaris squid from a local seafood restaurant and an online fishing bait store for dissection. We chose these species because the whole family of loliginid squid has been identified to contain reflectin, and these particular species were the only members of the family available to us.
We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following protocol. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli.
Squid Tissues
We set the microscope to collect light reflected from the sample (emission and collection wavelengths overlap) as we were searching for iridescence. We'd like to thank Paul Grant who optimised the settings on the microscope. We then overlaid the images ourselves to produce the animated gif on the right.
We are very grateful to Fernan Federici who helped us, taking the image below using the 405nm, 488nm, 633nm laser beams and with the pinhole opened to a wider aperture.
We used the enzyme trypsin to isolate cells from the mantle of the squid as this frees them from the extracellular matrix. Again we used the confocal microscope in a configuration that detects reflected light. Below is a non-iridescent cell from the tentacle imaged under the same conditions.
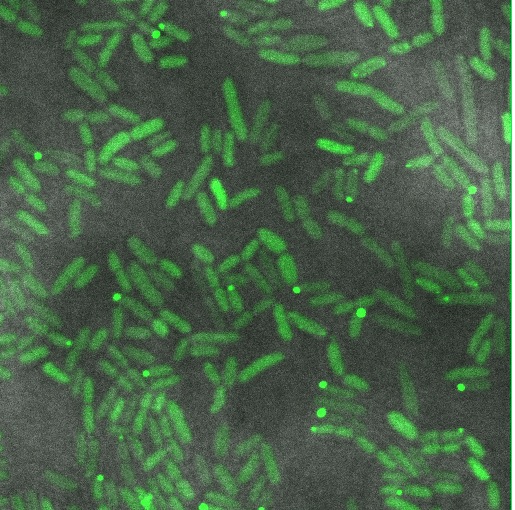
Reflectin Expressing Cells
These cells are producing a reflectin A1-GFP fusion protein from a high-copy plasmid (pSB1A3) showing fluorescent inclusion bodies.
We fused a TorA export sequence to a reflectin A1-green fluorescent protein fusion in an attempt to export reflectin to the periplasm of E coli. Unfortunately, even on a low copy plasmid, it appears that the export tag has failed or we have saturated the export pathway and a backlog has occured. This means all cells have a detectable amount of GFP in their cytoplasm and fluorescent inclusion bodies in several of the transformed cells.
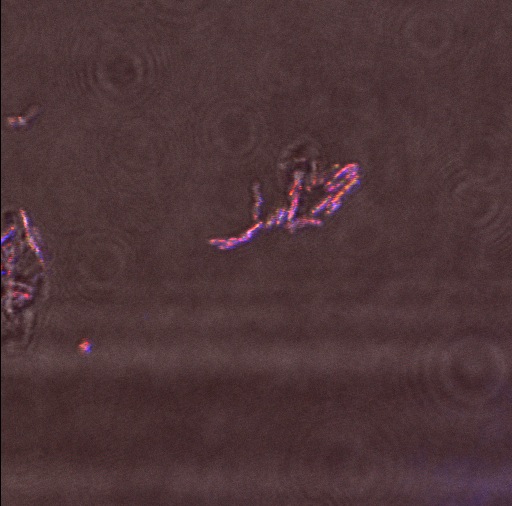
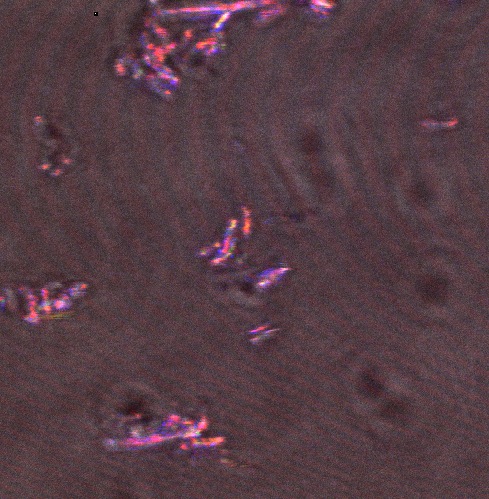
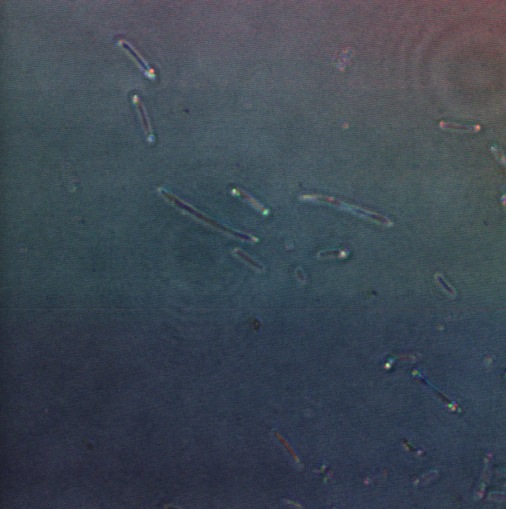
The following confocal micrographs show control bacteria, transformed but uninduced bacteria and bacteria containing our construct and induced to produce reflectin A1. Interestingly, all cells display some reflectance, and perhaps surprsingly the induced cells show the least overall reflectanct. The induced cells display punctate dots of reflectance, which suggests inclusion bodies have been formed. However, despite uniform imaging settings across the data set, there is a differing amount of reflectance from the medium the cells are growing on. Further work we would like to carry out would involve producing slides of the same bacteria but using gelatin and PBS to minimise the background reflectance, compare reflectin and non-reflectin inclusion bodies under various imaging techniques and make use of a spectrometer to gather spectral data on the reflectin expressing bacteria, as the limitations of reflected light confocal microscopy - namely background reflectance, is highlighted by these images.
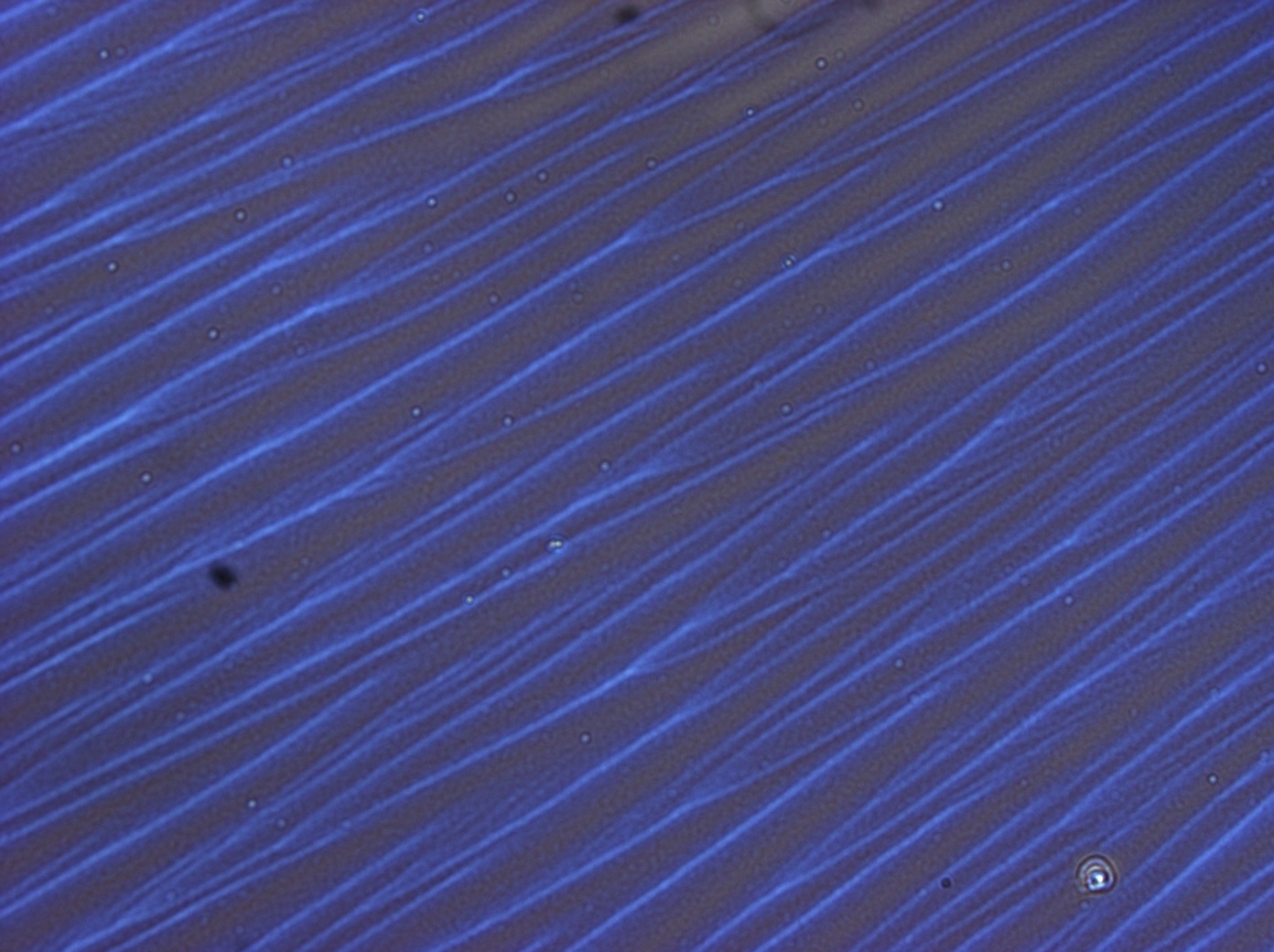
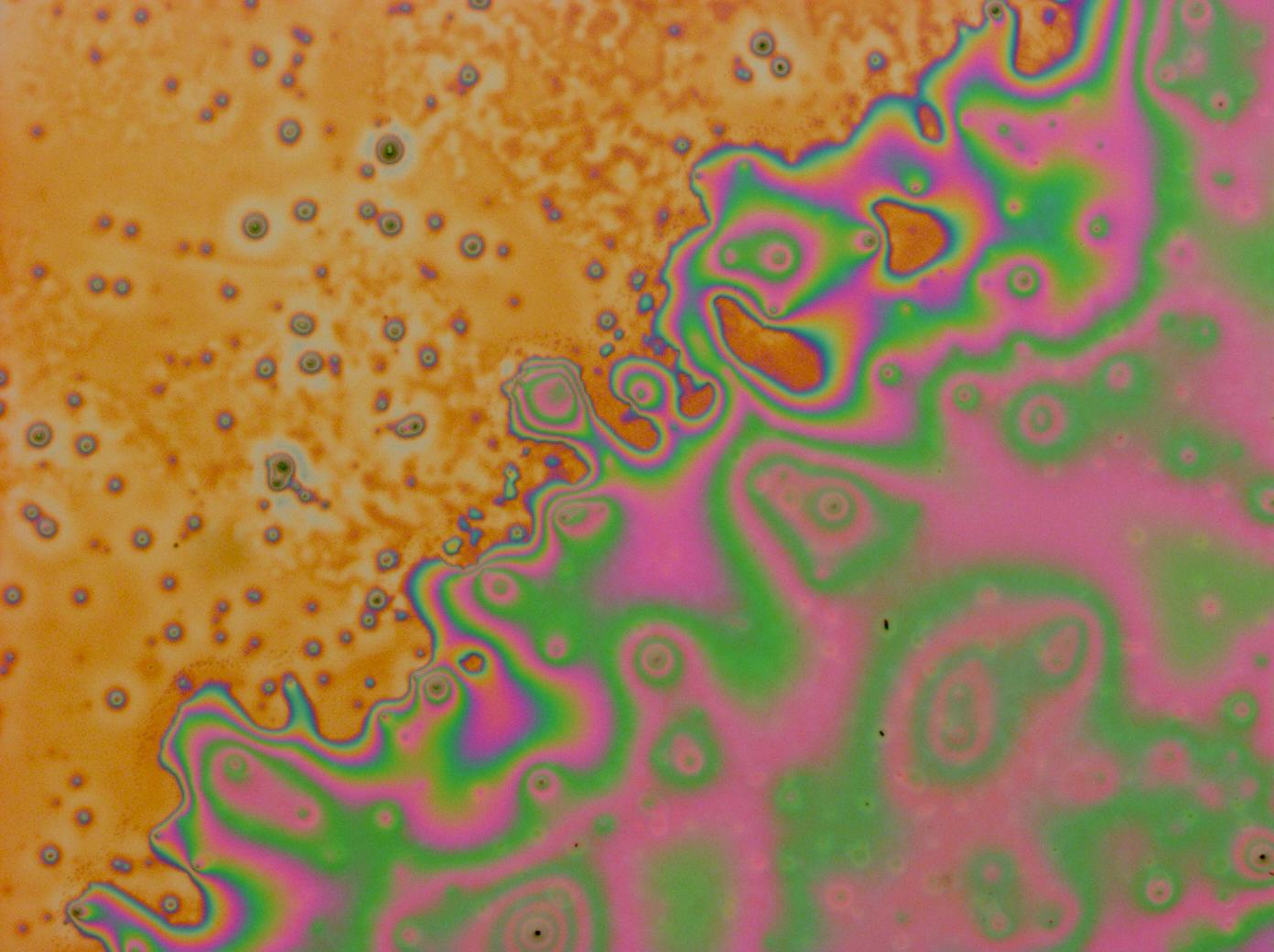
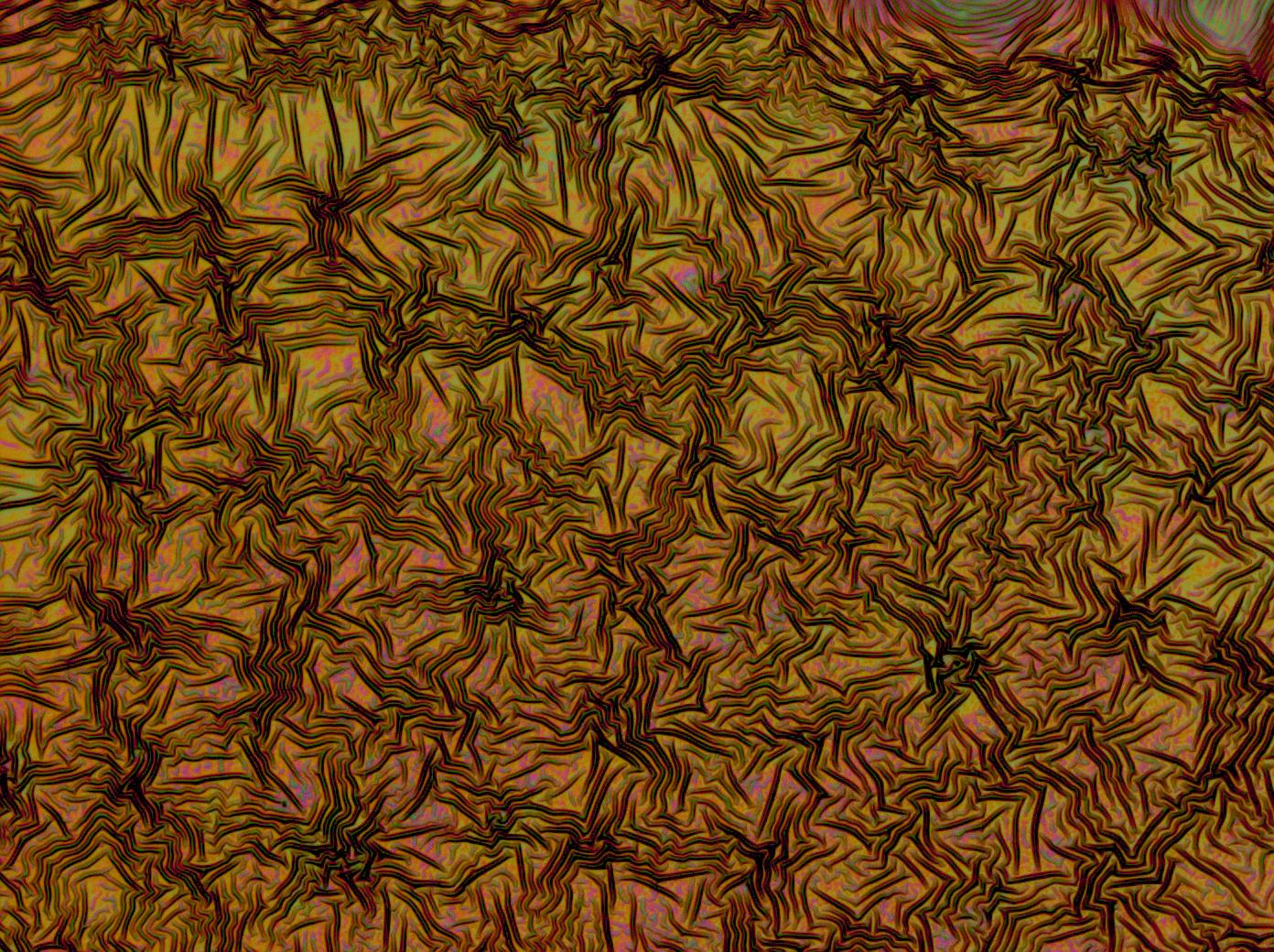
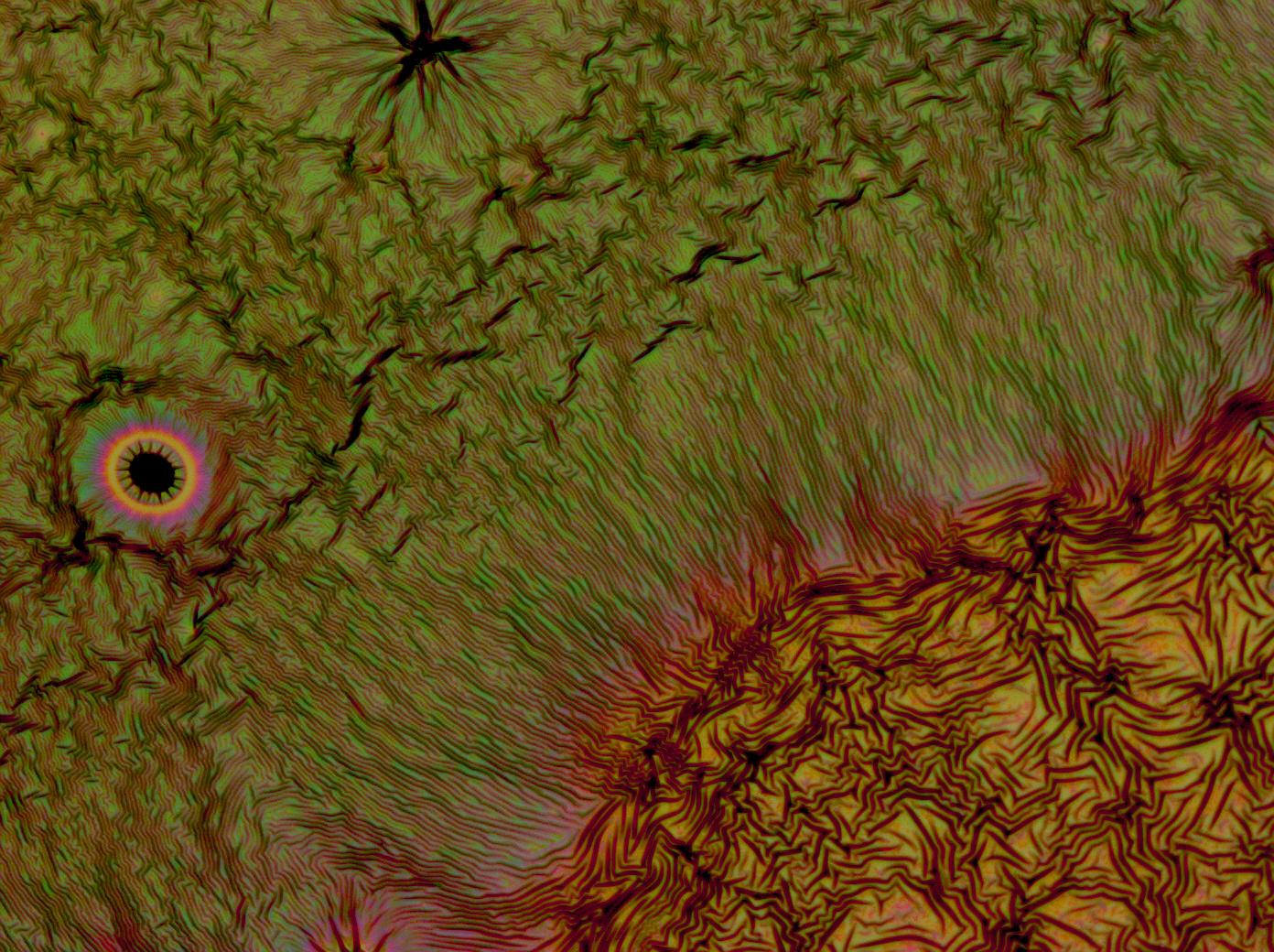
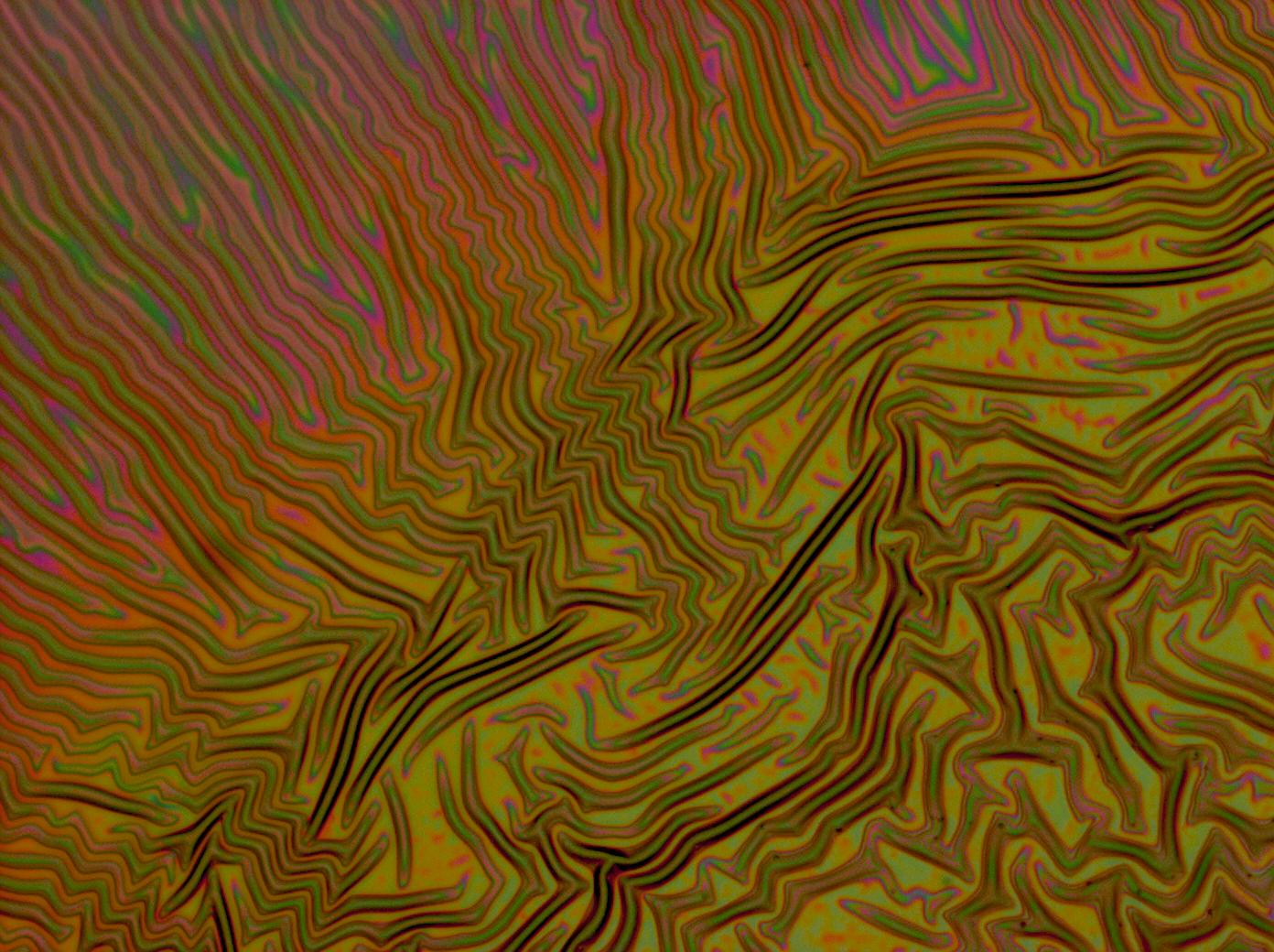
Reflectin Thin Films
Below is a selection of our thin film micrographs, including the relevant controls.
 "
"