Team:Cambridge/Safety
From 2011.igem.org
(→Containment) |
(→Safety inside the lab) |
||
| Line 10: | Line 10: | ||
==Safety inside the lab== | ==Safety inside the lab== | ||
| + | |||
| + | The proper waste disposal routes were followed stringently in order to prevent the reagents and biological material that we worked with from causing any harm to the environment or the general public. Within the lab, MSDS sheets were consulted whenever we planned to use new chemicals/reagents, to ensure that all possible safety hazards had been taken into consideration before any accidents could happen. The safety precautions that we took are all outlined in the description of our experimental methods in the [protocols section]. In this way, we managed to avoid any serious incidents from occurring over the summer. | ||
===Waste Disposal=== | ===Waste Disposal=== | ||
| Line 37: | Line 39: | ||
Since we will only be working with biosafety level one organisms, our work can be carried out on open bench tops, but gloves and lab coats must be worn during experimentation in order to protect the worker and reduce the risk of contamination. Within the lab, 'wet' experimental work was restricted to the 'Wet Area' whereas write-ups and computer work took place solely in the 'Dry Area'. Coats, bags, laptops and other belongings were also restricted to the Dry Area. These areas were clearly segregated to ensure nothing that left the lab could have come into contact with biological material at any point, further confining the biological material. | Since we will only be working with biosafety level one organisms, our work can be carried out on open bench tops, but gloves and lab coats must be worn during experimentation in order to protect the worker and reduce the risk of contamination. Within the lab, 'wet' experimental work was restricted to the 'Wet Area' whereas write-ups and computer work took place solely in the 'Dry Area'. Coats, bags, laptops and other belongings were also restricted to the Dry Area. These areas were clearly segregated to ensure nothing that left the lab could have come into contact with biological material at any point, further confining the biological material. | ||
All items which have come into contact with bacteria must be [https://2011.igem.org/Team:Cambridge/Safety#Autoclave autoclaved] before disposal, and neither lab-coats nor gloves should leave the laboratory. | All items which have come into contact with bacteria must be [https://2011.igem.org/Team:Cambridge/Safety#Autoclave autoclaved] before disposal, and neither lab-coats nor gloves should leave the laboratory. | ||
| - | |||
==Hazards for the Environment and the general public== | ==Hazards for the Environment and the general public== | ||
Revision as of 09:41, 25 August 2011
Safety is of utmost importance during projects involving synthetic biology. Not only must the welfare of scientist and non-scientific staff within the department be considered, but also that of the wider community. It is therefore important that safety procedures are in place to minimise the risk of harm to both employees and the wider community.
This has been implemented in our lab in many ways. We took care that everyone in the team was fully aware of all the safety protocols by attending a presentation given by our lab safety officer, Barbara Landamore, and reading the Department's Code of Practice for working with genetically modified (GM) organisms before being granted GM worker status.
Contents |
The Cambridge Biosafety Group
The biological safety officer for our department is Dr Ed Tanner. An interdepartmental team, the Biosafety Group has reviewed our project brief and has passed our nominations for all members of the team to be able to work with [http://en.wikipedia.org/wiki/Biosafety_level Biosafety level one] organisms in the Plant Sciences department, which is itself an approved containment level one facility. We have thus been cleared to work with bacteria that have been classified as being "unlikely to cause human disease". However, before carrying out any work with GM bacteria, the relevant risk assessment forms must be submitted to the Head of Group within the Department for approval.
Safety inside the lab
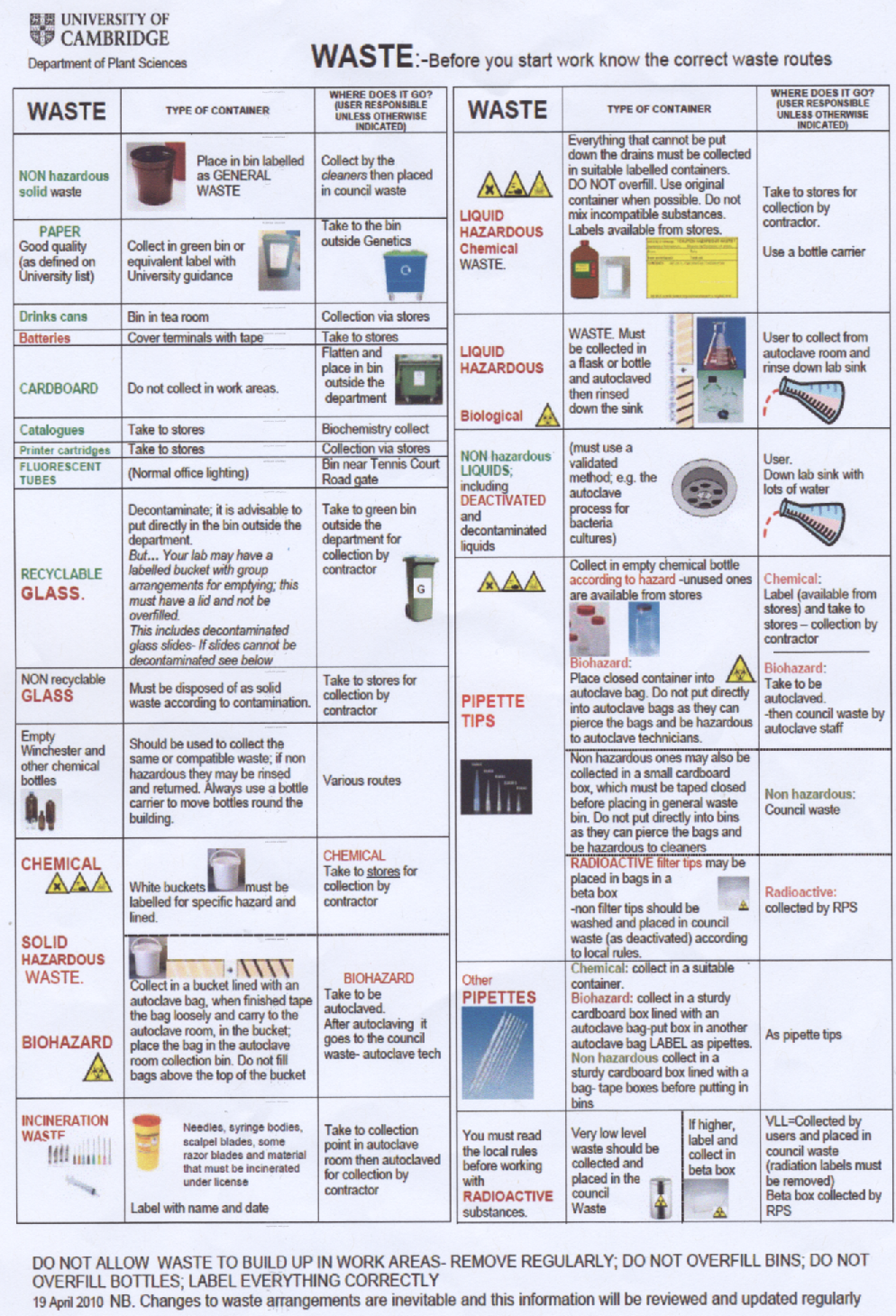
The proper waste disposal routes were followed stringently in order to prevent the reagents and biological material that we worked with from causing any harm to the environment or the general public. Within the lab, MSDS sheets were consulted whenever we planned to use new chemicals/reagents, to ensure that all possible safety hazards had been taken into consideration before any accidents could happen. The safety precautions that we took are all outlined in the description of our experimental methods in the [protocols section]. In this way, we managed to avoid any serious incidents from occurring over the summer.
Waste Disposal
Disposing of equipment used for experimental purposes is a significant challenge, and detailed procedures are in place within the department in order to ensure the safe disposal of all equipment, or its recycling where possible. The chart below outlines our departmental waste disposal policy - it is currently up on our wall in the lab.
Autoclave
All reasonable measures must be taken to prevent the release of biological material from the department, and so all biological matter must be autoclaved before disposal.
Glasses for autoclaving should be:
- Only partially filled: the solution will boil during autoclaving and hence expand
- Lidded (loosely) - gas must be able to escape (to prevent explosion)
- Labelled 'iGEM' so any problems can be reported back to us
- Left on the table to the right (see picture)
- Collected from the autoclaving room
Other equipment, e.g. petri dishes, should be bagged, labelled and placed in the yellow box to the right (see picture). If the items are sharp (and hence could penetrate the autoclave bags, presenting a hazard to autoclave technicians) then they should be placed in a secure closed container before being bagged.
Items which are unsuitably prepared for autoclaving (e.g. loose pipette tips in bags) wil be returned, and will not be autoclaved until they are prepared appropriately for the procedure.
Containment
Genetically modified material must not be allowed to leave the laboratory. While the likelihood of the strains of bacteria with which we will be working surviving in the wild is low, the environmental impact of such an event could be severe, and thus every reasonable precaution must be taken to prevent such an occurrence.
Since we will only be working with biosafety level one organisms, our work can be carried out on open bench tops, but gloves and lab coats must be worn during experimentation in order to protect the worker and reduce the risk of contamination. Within the lab, 'wet' experimental work was restricted to the 'Wet Area' whereas write-ups and computer work took place solely in the 'Dry Area'. Coats, bags, laptops and other belongings were also restricted to the Dry Area. These areas were clearly segregated to ensure nothing that left the lab could have come into contact with biological material at any point, further confining the biological material. All items which have come into contact with bacteria must be autoclaved before disposal, and neither lab-coats nor gloves should leave the laboratory.
Hazards for the Environment and the general public
We do not anticipate that any of the bacteria that we have worked with could pose any significant risks to the environment or the general public. The organisms are non-pathogenic, and not infective. They also survive only under very particular conditions that would be difficult to recreate outside the lab (i.e. pH, temperature, food). The bacteria should be unable to infect any living organism, since we used strains of E. coli that have been multiply disabled such that they can no longer survive in the human gut and did not (knowingly) expand its host range by genetic engineering. The reflectin protein that we produced is also known to be non-toxic, since it is present in the skin of many edible species of squid. However, since we are aware that unexpected risks may exist, we still ensured that any material that could potentially have come into contact with live bacteria were autoclaved before disposal. In this way, we maintained containment of the biological organisms within our lab.
Security Risks
We do not think that our genetically modified bacteria could be maliciously misused in any way that could pose a security risk, since (as described above) neither the organisms nor reflectin pose any notable hazards for the environment or the general public.
Working with reflectin
Since the DNA we will be working with originate from an edible squid, there should be no toxicity or pathogenic issues associated with working with reflectin. Culturing E. coli to produce reflectin also shouldn't expand it's host range (as far as we are aware), and we have no intention of releasing the GM organisms into either the human body or the environment, either during the competition or in speculative applications. Consequently, our project has minimal biosafety issues. This is no accident; these considerations featured heavily in our brainstorming sessions, and we discarded many potential project ideas because of their potential biosafety implications. This is the kind of practice we would like to encourage more iGEM teams to take seriously when choosing a project, since after browsing through past iGEM projects, it seems like many of the ideas would not have passed our stringent biosafety checks! Check out some of our suggested guidelines here.
We have not yet begun to develop specific experimental plans, but when we do we will submit detailed risk assessments to the relevant authority before we commence our work. The associated safety issues will be discussed in the experiments section. Similarly, we haven't yet created any new BioBricks, but we will discuss the relevant biosafety considerations as soon as they arise. Should an organism containing the reflectin expressing gene escape from the lab, we do not anticipate any significant risks to the public or the environment since we do not intend to increase the host range of the host bacteria, nor alter its pathogenicity. Since we will be working with multiply disabled strains of E. coli (which cannot survive in the gut, unlike pathogenic strains), this should render our bacteria effectively harmless.
Protocols
The safety issues associated with common protocols that we employ are/will be detailed in the protocols section. As with everything else on this wiki, it's still a work in progress!
 "
"