Team:Cambridge/Experiments/Reflectin Thin Films VI
From 2011.igem.org
Felix Zhou (Talk | contribs) (→Results) |
Felix Zhou (Talk | contribs) (→Temporary Solution) |
||
| (8 intermediate revisions not shown) | |||
| Line 43: | Line 43: | ||
===Results=== | ===Results=== | ||
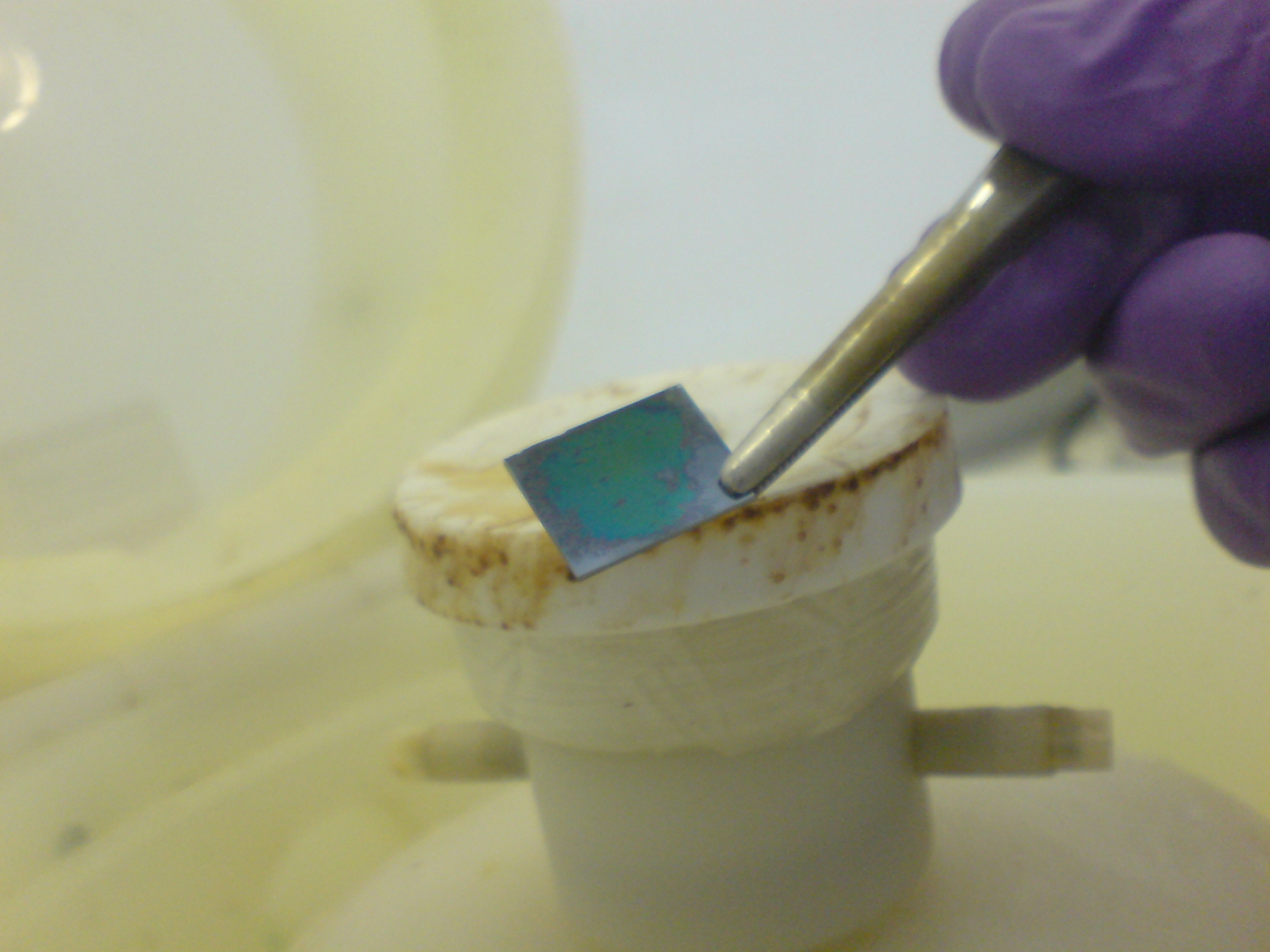
| + | [[File:cam_blue_thin_dialyse_good.jpg|thumb|top|150 px|Blue film from dialysed proteins as you can see the surface is riddled with spots where the proteins seem to have beaded up but it illustrates the potential of these films as an alternative to acetone precipitation]] | ||
| + | [[File:cam_blue_thin_dialyse_awesome.jpg|thumb|bottom|150 px|Blue film from dialysed proteins as you can see the surface is riddled with spots where the proteins seem to have beaded up but it illustrates the potential of these films as an alternative to acetone precipitation]] | ||
| + | |||
None of the above suggestions yielded very good results. | None of the above suggestions yielded very good results. | ||
| Line 51: | Line 54: | ||
===Temporary Solution=== | ===Temporary Solution=== | ||
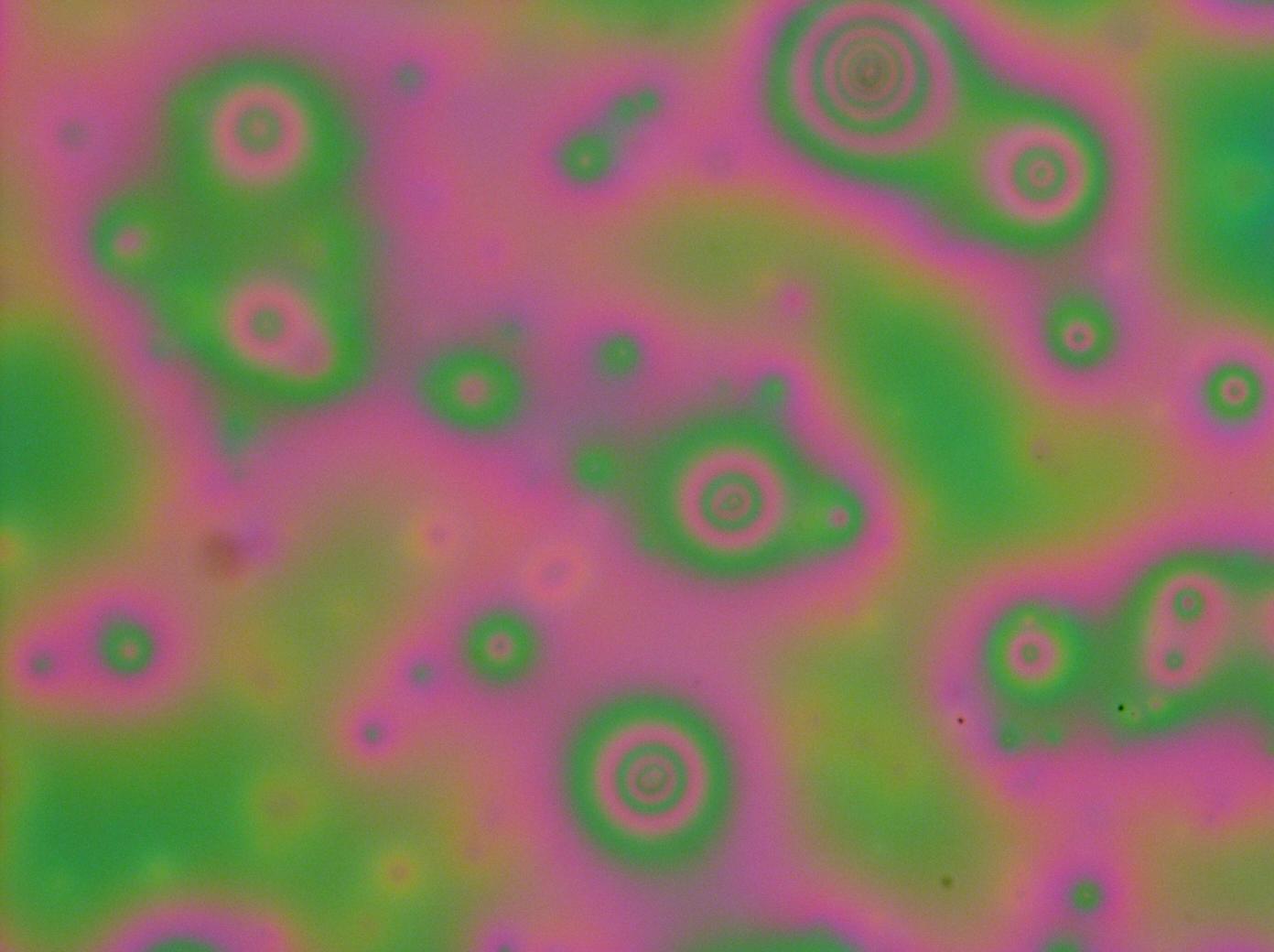
| - | Whilst not perfect dialyzed proteins provide an avenue for the investigation of multilayers as instead of crystallizing they simply dewet and lose colouration. Though not ideal the produced films were good enough for us to take [https://2011.igem.org/Team:Cambridge/Experiments/Reflectin_Thin_Films_VII#/Experiments/Reflectin_Thin_Films_VII spectral measurements] at 20x objective lens and for us to demonstrate the tunability of reflectin thin films by water vapours through breathing. | + | [[File:cam_multilayer_20x.jpg|thumb|left|200px|Close up microscopy with 20x objective lens at successful 3 layer multilayer. As can be seen there are many defects in the form of bumps]] |
| + | Whilst not perfect dialyzed proteins provide an avenue for the investigation of multilayers as instead of crystallizing they simply dewet and lose colouration. Though not ideal the produced films were good enough for us to take [https://2011.igem.org/Team:Cambridge/Experiments/Reflectin_Thin_Films_VII#/Experiments/Reflectin_Thin_Films_VII spectral measurements] at 20x objective lens and for us to demonstrate the tunability of reflectin thin films by water vapours through breathing. However you can see from the microscope images they are far from perfect! and much more work needs to be done in following weeks. | ||
===Conclusion=== | ===Conclusion=== | ||
Latest revision as of 03:55, 22 September 2011
Contents |
Attempts to increase film stability lead to first stable multilayer
Modifying Protein Concentration Methods
Need for Improving Protein Impurity
Protein impurity has become a major headache and hindrance on our progress. If we acetone precipitate to concentrate then whilst there is no dewetting phenomenon and concentration yields are high, films are dominated by the strong aggregation tendency of urea to crystallize. Meanwhile, alternatively if we use dialysis in 10mM Tris, 0.1% Triton-X 100 pH 8.0 buffer in dialysis membrane, there are issues with tris preventing efficient solvent evaporation and wetting which lead to degradation and dewetting of the films which lead to in many cases non-uniformity and loss of colouration over time.
Alternative protein concentration methods were tried in order to help purity:
- Chloroform/methanol/water precipitation
- Microcentrifuge dialysis against 10mM Tris, 0.1% Triton-X 100 pH 8.0 buffer followed by vacuum centrifuge
- Microcentrifuge dialysis against 1X PBS buffer and pH 8 buffer followed by vacuum centrifuge
- Microcentrifuge dialysis against unbuffered D.I water followed by acetone precipitation
Samples from each method were then first drop cast onto silicon wafer to test for presence of protein and subsequently spun onto silicon substrate after dilution with 10-40 μl of HFIP with volume differing based on my expectation of protein yield.
Results
The results from this experiment shows that:
- Chloroform/methanol/water precipitation was too effective, not only did it get rid of contaminants it also removed the protein! However this outcome was within our calculations. From hydropathy plots Crookes et al determined that Loligo Reflectin A1 is a globally hydrophilic protein therefore there may be a possibility of it associating two strongly with the aqueous phase and therefore not precipitating in the interphase. Nevertheless I would still have expected more protein than nearly nothing, this I speculate is because the protocol should be implemented with all reagents at ice-cold temperatures to facilitate precipitation. We intend to repeat this protocol with ice cold reagents.
- Microcentrifuge dialysis though yielded much greater concentration of proteins than using the entire membrane was not as effective and much of the urea salt was retained in the microcentrifuge. Upon taking a sample of the crystals from the Tris buffer dialysis, diluting in 40μl HFIP and spinning at 3000 rpm for 1 min with 2μl of the reflectin-HFIP solution on silicon substrate one of the most beautiful films I have produced was seen. The film was a brilliant shade of green of perfect uniformity and thick enough to exhibit iridescence from different viewing angles, an effect seen more prominently when coated onto a layer of PDMS. Unfortunately soon after we found that the films crystallised indicating much of the resulting crystals after vacuum centrifuge are urea crystals. The brilliant colouration and wetting is believed to be the high concentration of proteins which should be close to the eluted concentration.
- The result of the dialysis with 1X PBS buffer again was not impressive with crystallisation within 10 seconds of spin coating and almost instantly when drop-casting making it unfeasible to work with.
- The result of unbuffered water too was not brilliant with very little iridescence after drop-casting.
Attempting to Stabilise Films
Hypothesizing from Experience
Based on our many different attempts to spin uniform, stable films we have noticed the following points which indicate some potential in at least delaying the onset of crystallizing.
- Crystallization is accelerated by heat
- Crystallization is promoted by greater solvent evaporation
- Crystallization takes much longer when using the first 2μl of a centrifuged reflectin-HFIP sample but rapidly got worse in the next few aliquots which suggests centrifugation may be fractionating some urea.
Proposed Methods
Several measures were investigated based upon the above observations:
- Dilution with more HFIP which provides a greater volume of fluid improving the possibility of contamination from the pellets produced in centrifugation. This was a technique used in the dialysed proteins to reduce the amount of insoluble tris contaminants.
- Attempting to filter the solutions particularly for the dialyzed proteins however the small working volumes makes this unreasonable
- Manually stopping the spin coater to leave sufficient solvent to delay crystallisaton and dewetting.
- Coat PDMS layer immediately after spin to not leave enough time for cystallisation or dewetting due to local humidity.
Results
None of the above suggestions yielded very good results.
- Greater dilution meant less concentrated proteins therefore diluting too much will not leave a film and the greater proportion of solvent led to evaporation problems which meant often samples had to be hotplated which for acetone precipitated films meant promoting crystallisation whilst with dialysed proteins meant accelerating dewetting.
- With the yields we were getting filtering was always going to be unfeasible. The one time we did filter, we combined several different samples to get approximately 160μl of solution which was filtered with a millipore 0.22 μm filter tip using a syringe. The spun-cast films were of much higher quality for the dialysed proteins however improvements were not long lasting and does not tackle the fundamental problem.
- Manually stop though may be feasible is very difficult to judge both stopping too soon and stopping too late were found to be bad. Too soon and not enough solvent has evaporated and the layer is not sufficiently uniform, too late and the film will already have crystallised or begun dewetting. Furthermore the narrow range of speeds over which could potentially search for given quality varying proteins and limited spins and time did not lend much hope here either.
- Coating with PDMS initially seemed good when the layer was hydrophilic but after the second layer, the PDMS will revert to its previous hydrophobic state whilst the reflectin layer is hydrophilic therefore will simply compound the stability of the reflectin films even more and this is exactly what was observed in the form of protein art for acetone precipitated proteins.
Temporary Solution
Whilst not perfect dialyzed proteins provide an avenue for the investigation of multilayers as instead of crystallizing they simply dewet and lose colouration. Though not ideal the produced films were good enough for us to take spectral measurements at 20x objective lens and for us to demonstrate the tunability of reflectin thin films by water vapours through breathing. However you can see from the microscope images they are far from perfect! and much more work needs to be done in following weeks.
Conclusion
- More research and work into protein concentration methods with the possibility of purchasing commercial kits
- In the meantime continue to work with dialyzed proteins which is more stable than the acetone precipitated proteins and allows for investigating into improving the fabrication of multilayers though ultimately the quality of this will naturally improve with greater concentration and purity of proteins.
Back to Experiments
 "
"