Team:Cambridge/Project/In Vitro
From 2011.igem.org
(→Protein extraction and purification) |
(→Tagging the reflectin gene) |
||
| Line 9: | Line 9: | ||
==Tagging the reflectin gene== | ==Tagging the reflectin gene== | ||
| - | The reflectin genes were his-tagged by incorporating the | + | The reflectin genes were his-tagged by incorporating the sequence of part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K128005 BBa_K128005] into the primers used to clone the gene. His tagging was chosen because it allows proteins to be purified with relatively simple apparatus, a plastic column, to which a metal containing resin is added traps the protein when cell lysate is added. |
==Overexpression== | ==Overexpression== | ||
Revision as of 21:54, 18 September 2011
Contents |
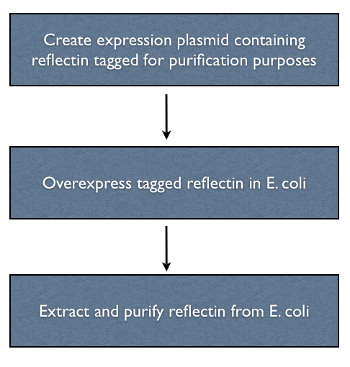
Objective Two - Isolate and Purify Reflectin in vitro
Purification of recombinant reflectin from bacteria was necessary in order to perform all of the subsequent in vitro studies of the protein. An over-exressing vector construct was used to maximise yield, and a his-tag was used for purification purposes.
Tagging the reflectin gene
The reflectin genes were his-tagged by incorporating the sequence of part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K128005 BBa_K128005] into the primers used to clone the gene. His tagging was chosen because it allows proteins to be purified with relatively simple apparatus, a plastic column, to which a metal containing resin is added traps the protein when cell lysate is added.
Overexpression
We chose to use a high copy plasmid with a pBAD promotor in order to overexpress reflectin. This produces inclusion bodies, which will create unfolded reflectin, but this is not a concern because the solvents
Protein extraction and purification
A number of protein purification protocols were used to isolate the reflectin-containing inclusion bodies from transformed E. coli.
The Norgen Proteospin inclusion body prep. kit was experimented-with as a commercial alternative to the ethanol/acetone precipitations used previously. The kit is simple to use, but does require access to an ultracentrifuge capable of spinning up to 25ml of fluid at 27,000g. The latter stages of the protocol require numerous uses of a benchtop centrifuge. It was found that the spinning forces specified in the protocol for these steps weren't capable of moving fluid through the necessary columns and higher forces were needed. On analysing the end-product of the purification with SDS-PAGE approximately 20 discrete bands were observed from 10-200 kda with 2 bold bands, one of which corresponded to the size of reflectin.
 "
"