Team:Brown-Stanford/REGObricks/Biocementation
From 2011.igem.org
Maxsong123 (Talk | contribs) (→Biocementation) |
Maxsong123 (Talk | contribs) (→More about Urease) |
||
| Line 39: | Line 39: | ||
Pathogenesis of Helicobacter pylori Infection | Pathogenesis of Helicobacter pylori Infection | ||
Clin. Microbiol. Rev., Jul 2006; 19: 449 - 490. | Clin. Microbiol. Rev., Jul 2006; 19: 449 - 490. | ||
| + | |||
BE Dunn, NB Vakil, BG Schneider, MM Miller, JB Zitzer, T Peutz, and SH Phadnis | BE Dunn, NB Vakil, BG Schneider, MM Miller, JB Zitzer, T Peutz, and SH Phadnis | ||
Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies | Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies | ||
Revision as of 06:07, 26 September 2011
Biocementation
What is Biocementation?
“Biocementation” is the growing field of employing biological agents as catalysts for increasing the stiffness and strength of soil [1]. The particle branch of biocementation that we investigated in our project this summer was “Microbial Induced Carbonate Precipitation (MICP)”, a process by which microorganisms metabolize various nitrogen products to increase ambient pH. The salient organism in our case is Sporosarcina pasteurii (previously Bacillus pasteurii), which utilizes the hydrolysis of urea to generate carbon dioxide and ammonia, which ultimately convert to carbonate ions in a basic environment. Carbonate fuses with available metals in solution to precipitate in crystal formation, and fuse surrounding particles together.
The Chemical Reaction
Urease (urea amidohydrolase; EC 3.5.1.5) is a six-subunit protein assembled from three alpha and three beta subunits.
Contained in the center of the urease enzyme is dual nickel metallocore, crucial to its activity (Mobley HL et al). Studies varying the concentration of nickel show a strong dependence on the availability of metal anions; mutations that remove the expression of an accessory metal-transport protein leads to dramatic decreases in urease activity []. During each cycle of activity, urease breaks down one urea molecule into one molecule of ammonia and one molecule of carbamate, which is unstable and decomposes to another molecule of ammonia and bicarbonate. Each of the ammonia molecules have a pKa of 9.31, and take up protons from the cytoplasm. They are then shuttled out of the cell to create an proton gradient for ATP-generation. Outside the cell, ammonium contributes to an increase of pH in the surrounding environment. The cell itself also serves as a nucleation site, as positively charged calciums in solution are attracted by its negative membrane. The gradual evolution of carbonate from the basic environment begins the process of calcium precipitation, and the rest, they say, can be seen in stone!

The genetic sequence of the urease has been ascertained for several strains of bacteria, including H. pylori, and some partial mappings have been done for individual subcomponents of S. pasteurii [] []. There have also been occasional mentions of strains of recombinant bacteria containing urease function [] [] [] though unfortunately, none currently in a biobricked form. While the study of urease function in H. pylori has matured under medicinal interest over the years, the genetic expression of urease is still a field of ongoing research. Ambitiously, we decided that one of the project goals for REGObricks would be to curate an modular, enzymatically functional urease to the iGEM registry of Standardized Parts. (link here) __________________________________
More about Urease
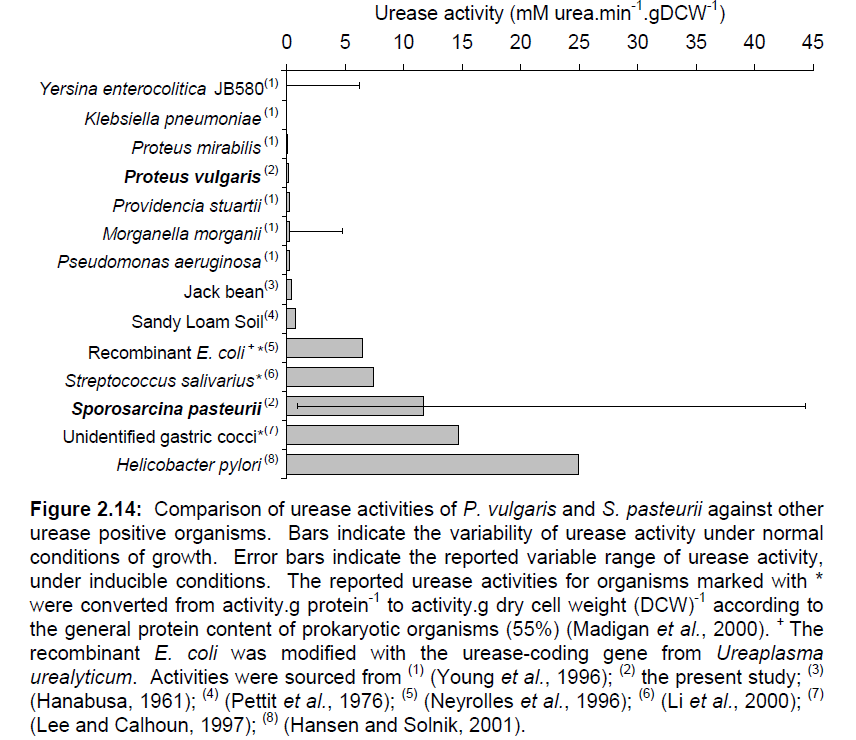
Iconic in the history of protein science, urease was the first enzyme to be purified by James Sumner from a jack bean plant in 1926 [2]. Since then, urease has been well-known in the medical field as a convenient way to test for the presence of urinary tract pathogenetic bacteria such as Helicobacter pylori, one of the chief culprits in the formation of duodenal ulcers and stomach cancer. The strength and enzymatic activity of the enzyme is distributed across a wide range of species, reflecting the different functions it serves. A survey of some representative species of bacteria is given in Figure 1. Note S. pasteurii’s sustentative range of urease activity.
Continue on to our characterization of S. pasteurii!_____________
CITATIONS______________________
Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59(3):451–480. [PMC free article] [PubMed] Dependence of Helicobacter pylori Urease Activity on the Nickel-Sequestering Ability of the UreE Accessory Protein http://jb.asm.org/cgi/content/full/185/16/4787 Johannes G. Kusters, Arnoud H. M. van Vliet, and Ernst J. Kuipers Pathogenesis of Helicobacter pylori Infection Clin. Microbiol. Rev., Jul 2006; 19: 449 - 490.
BE Dunn, NB Vakil, BG Schneider, MM Miller, JB Zitzer, T Peutz, and SH Phadnis
Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies Infect. Immun., Apr 1997; 65: 1181 - 1188. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J. Bacteriol., Apr 1992; 174: 2466 - 2473. David L. Weeks, Sepehr Eskandari, David R. Scott, and George Sachs A H+-Gated Urea Channel: The Link Between Helicobacter pylori Urease and Gastric Colonization Science, Jan 2000; 287: 482 - 485.
 "
"