Team:Amsterdam/Notebook/Protocols/ColonyPCR
From 2011.igem.org

Colony PCR
We used this protocol to check the new transformants.
Materials
- Petri dish with LB agar and appropriate antibiotic
- ON colonies on a petri dish with LB agar and appropriate antibiotic
- dNTPs
- primers ([http://partsregistry.org/Part:BBa_G00100 VF2] and [http://partsregistry.org/Part:BBa_G00101 VR])
- Taq polymerase
- Taq buffer
- dH2O
- PCR tubes
- PCR machine
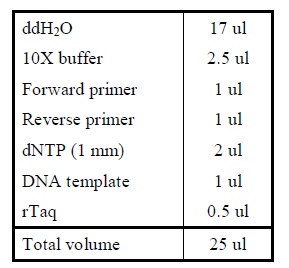
Protocol
| Sample | µl |
|---|---|
| VF2 primer | 0,5 |
| VR primer | 0,5 |
| dNTPs | 1 |
| Taq buffer | 2,5 |
| Taq polymerase | 0,2 |
| H2O | 20,3 |
| Total | 25 µl |
- Make the PCR mix and pippete it in a PCR tube.
- Pick a colony from a LB-agar plate using a sterile pipette tip.
- Pippete up and down in the PCR mix.
- Cross the pippete tip on the new LB-agar plate in the box corresponding to the sample number.
- Redo this with all the selected colonies.
- Run the following PCR program:
Note: Adjust the elongation time according to the expected product size. 1 minute for each Kb.
- Incubate the selected colonoies on the LB-agar plate ON at 37°C.
- Check the PCR-sample sizes on agarose gel.
 "
"














