Team:Brown-Stanford/PowerCell/Cyanobacteria
From 2011.igem.org
Ho.julius.a (Talk | contribs) (→Nitrogen and Carbon Dioxide) |
Ho.julius.a (Talk | contribs) (→Transformation of Cyanobacteria) |
||
| Line 65: | Line 65: | ||
Another problem is the propensity of Anabaena to slice and dice foreign DNA with isoschizomers of the restriction enzymes AvaI, AvaII and AvaIII. This has been addressed with methyltransferases targeting the same sequences; yes, that means a third E. coli strain carrying these methyltransferases (a helper plasmid) participates in the conjugation. At the end of all this, a certain number of cyanobacterial cells take up the DNA, and are selected for with neomycin on minimal media. As soon as the unsuccessful exconjugates and the bacterial parental strains die off, transformant colonies can be picked. This is best started well ahead of any sort of deadline—the transformants can take upwards of a week to grow. | Another problem is the propensity of Anabaena to slice and dice foreign DNA with isoschizomers of the restriction enzymes AvaI, AvaII and AvaIII. This has been addressed with methyltransferases targeting the same sequences; yes, that means a third E. coli strain carrying these methyltransferases (a helper plasmid) participates in the conjugation. At the end of all this, a certain number of cyanobacterial cells take up the DNA, and are selected for with neomycin on minimal media. As soon as the unsuccessful exconjugates and the bacterial parental strains die off, transformant colonies can be picked. This is best started well ahead of any sort of deadline—the transformants can take upwards of a week to grow. | ||
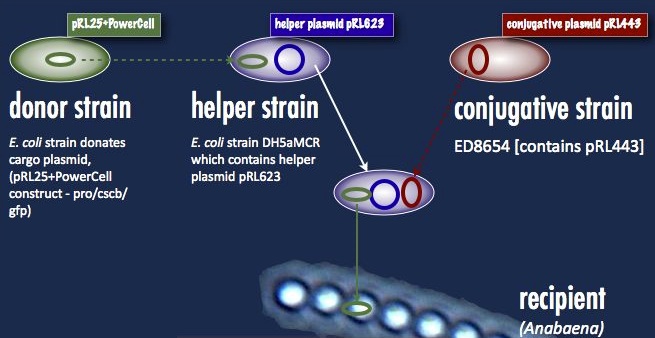
| - | [[File:Brown-Stanford Triparental mating.JPG|300px|thumb|Triparental mating: Our desired construct (from the '''donor strain''') and a helper plasmid are inserted into a '''helper strain'''. The '''helper strain''' and '''conjugative strain''' are spotted with the '''recipient''' Anabaena for the three-parent mating]] | + | [[File:Brown-Stanford Triparental mating.JPG|300px||center|thumb|Triparental mating: Our desired construct (from the '''donor strain''') and a helper plasmid are inserted into a '''helper strain'''. The '''helper strain''' and '''conjugative strain''' are spotted with the '''recipient''' Anabaena for the three-parent mating]] |
| - | + | ||
===References=== | ===References=== | ||
Revision as of 07:51, 28 September 2011
CRITERIA: CARBON FIXING, NITROGEN FIXING, MINIMAL MEDIA, ACCEPT DNA (WHY ARE EACH OF THESE IMPORTANT?)
BRIEF EXPLANATION OF PHOTOSYNTHESIS: CHEMICAL EQUATION, PICTURE OF EARTH CARBON CYCLE
DIAZOTROPHY: EXPLAIN WHAT IT IS, SHOW EARTH NITROGEN CYCLE, INTRODUCE PROBLEM OF OXYGEN AND NITROGENASE ACTIVITY -TWO METHODS OF DEALING WITH OXYGEN PROBLEM: UNICELLULAR TEMPORAL CONTROL AND HETEROCYSTS
ON THIS BASIS, WE SELECTED ANABAENA.
ANABAENA IS GROWN ON MINIMAL MEDIA, ACCEPTS DNA (JUST CITE A PAPER AND SAY WE WILL ELABORATE ON THIS PROCESS IN OUR EXPT SECTION)
Cyanobacteria
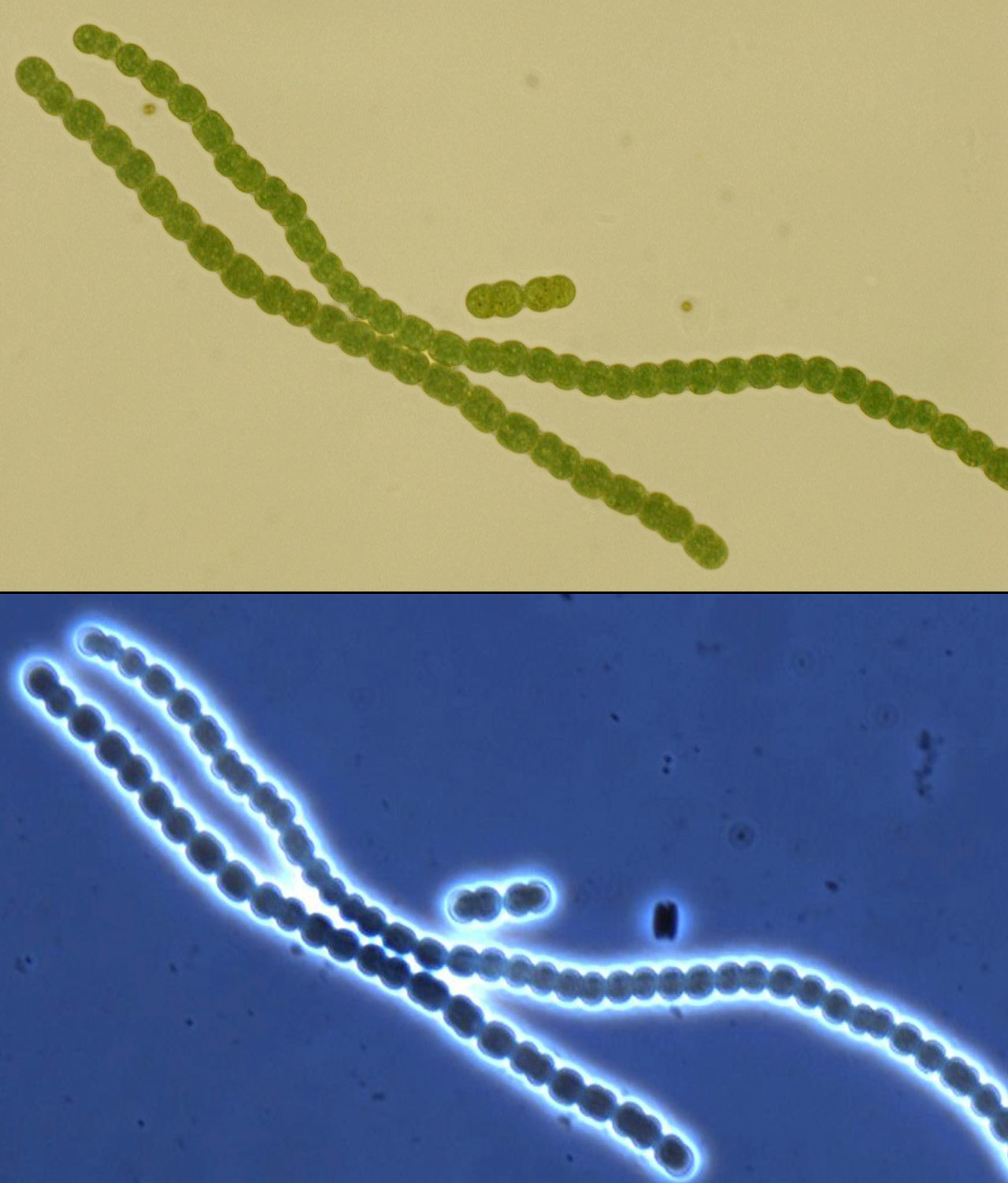
The search for a suitable chassis on which to build the PowerCell project turned up several promising contenders: algae are very efficient solar powerhouses, and E. coli has been engineered to express rubisco and perform a rudimentary form of carbon sequestration, among others. After carefully weighing our options, we hit upon cyanobacteria, commonly (and slightly inaccurately) called blue-green algae. At the end of our deliberations, the grand winner was Anaebaena PCC 7120, a filamentous organism very much resembling a tiny, green, pearl necklace.
The reasons for our choice were severalfold. Our primary requirements were that the organism access nitrogen and carbon dioxide, and accept our DNA in some relatively easy manner. These will each be explained in greater detail in sections below. In addition, we wanted an organism that could survive on minimal media, produce all of its products in aerobic conditions, and live a happy, fulfilled life with as little auxiliary equipment as possible.
Nitrogen and Carbon Dioxide
Anaebaena is a diazotroph, or “N2 eater.” The organism has evolved a nitrogenase which is capable of overcoming the considerable triple bond between the two nitrogen atoms, although the reaction can only take place in an anaerobic environment.
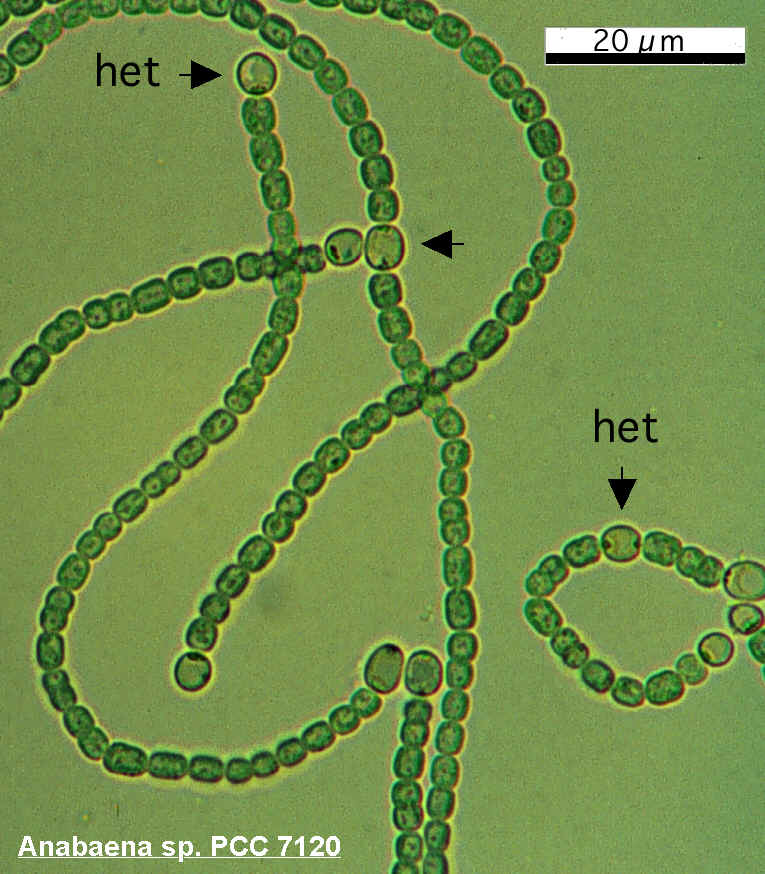
For this reason, many diazotrophic cyanobacteria require anaerobic growth conditions, but Anabaena has evolved a method of separating oxygen and the N2 cleaving process; specialized cells, called heterocysts, form along the filament in nitrogen-poor conditions. These specialized cells, visibly larger and darker than the rest, form an anaerobic intracellular microenvironment where the nitrogenase can do its job. The nitrogen-containing products are exported to adjacent vegetative cells via plasmodesmata, through which photosynthesized carbon products return.
Anabaena can live in aerobic conditions, fix nitrogen and photosynthesize sugars. They are able to provide almost everything they need, making them capable of living on very minimal media—clear water with a few trace minerals. It follows that we can harness their self-sufficiency to provide for more dependent organisms, such as E. coli. All we have to do is enforce some compulsory generosity, and although Anabaena won’t be as well-fed as it was as a selfish microbe, the E. coli it is supporting will have a food source where it otherwise would have gone hungry.
Transformation of Cyanobacteria
There are cyanobacteria which will accept DNA without complaint. Synechocystis elongatus, for example, will simply take up naked DNA in solution and express it. Anabaena, although it has been transformed, must take its DNA through a rather circuitous path; the DNA construct must first be placed in a cargo plasmid and transformed into E. coli by traditional means. Transfer to Anabaena takes place by conjugation, facilitated by a second E. coli strain carrying a plasmid encoding the machinery for bacterial conjugation.
Another problem is the propensity of Anabaena to slice and dice foreign DNA with isoschizomers of the restriction enzymes AvaI, AvaII and AvaIII. This has been addressed with methyltransferases targeting the same sequences; yes, that means a third E. coli strain carrying these methyltransferases (a helper plasmid) participates in the conjugation. At the end of all this, a certain number of cyanobacterial cells take up the DNA, and are selected for with neomycin on minimal media. As soon as the unsuccessful exconjugates and the bacterial parental strains die off, transformant colonies can be picked. This is best started well ahead of any sort of deadline—the transformants can take upwards of a week to grow.
References
[1] http://chemwiki.ucdavis.edu/index.php?title=Wikitexts/UC_Davis/UCD_Chem_124A:_Berben/Nitrogenase/Nitrogenase_2&bc=0
 "
"