Team:British Columbia/Notebook/Week 20
From 2011.igem.org
(→Synthetic Biology Gone Wild: Expert Opinions) |
|||
| Line 154: | Line 154: | ||
To obtain expert opinions on releasing synthetic organisms in the wild and initiate discourse on this topic, our team interviewed many experts across numerous fields at UBC. | To obtain expert opinions on releasing synthetic organisms in the wild and initiate discourse on this topic, our team interviewed many experts across numerous fields at UBC. | ||
| - | For the interview transcripts and some of the key take-home messages, please view [[British Columbia/Wild|Expert Opinions on Synthetic Biology Gone Wild]]! | + | For the interview transcripts and some of the key take-home messages, please view [[Team:British Columbia/Wild|Expert Opinions on Synthetic Biology Gone Wild]]! |
Revision as of 05:43, 28 October 2011

 |
 |
 |
 |
 |
Contents |
Diterpene production in yeast with LAS and ISO
We obtained four yeast strains from Katrin and Niels (from Bohlmann lab).
The yeast strains contained
- Levoabietadiene synthase + CYP720B4
- Levoabietadiene synthase
- Isopimaradiene synthase + CYP720B4
- Isopimaradiene synthase
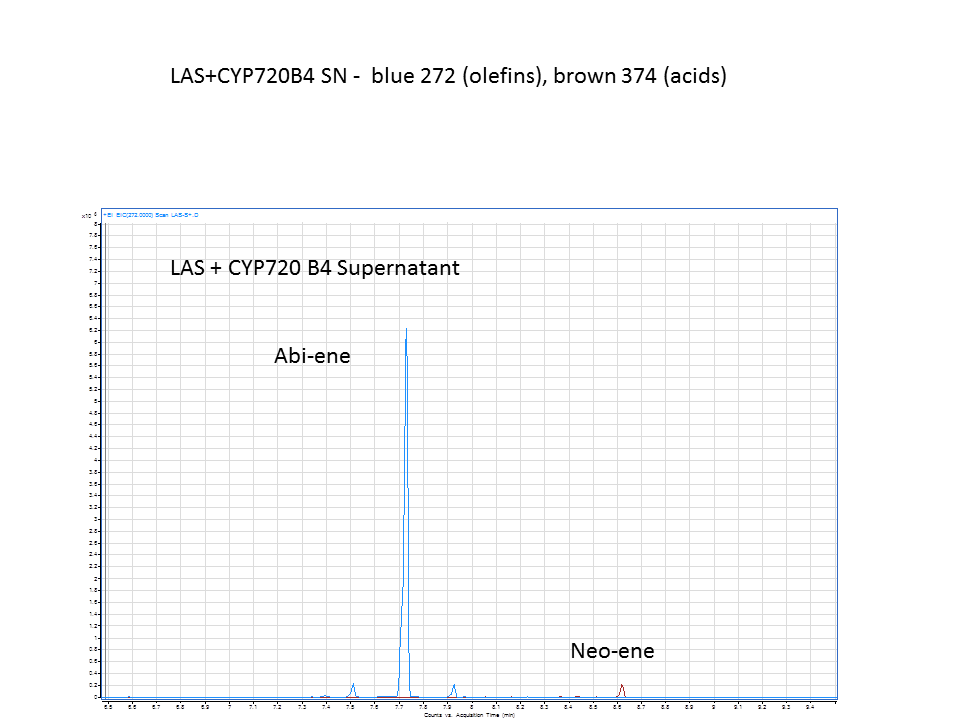
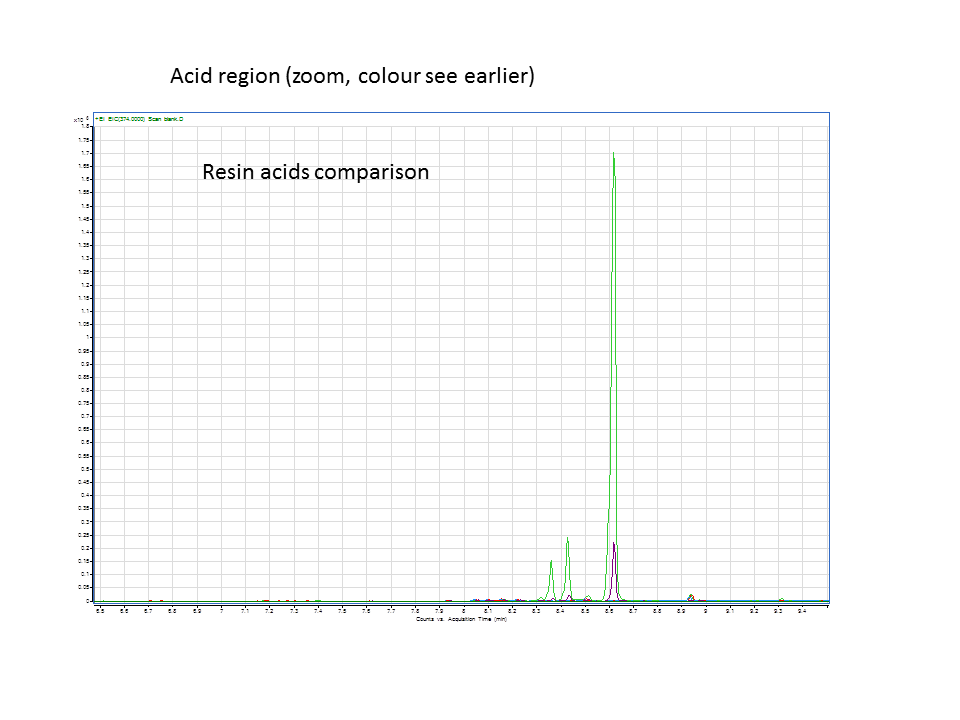
These are diterpene synthases which convert GGPP to the diterpinols, which are hydrolyzed to form diterpenes. These diterpenes can be further hydroxylated to form resin acids. CYP720B4 is an enzyme isolated by members of Bohlmann lab. This enzyme plays a key role in the production of specific acids that have been shown to have anti-white pine weevil effects.
The goal is to express diterpenes and show secretion into the media.
LAS/ISO were on pESC-HIS. GGPP synthase is also on the same plasmid as the synthases.
CYP720B4 is on another vector, -URA marked.
These experiments were conducted twice.
We obtained the protocol from Anne (Thanks anne!). There was also an extra derivitization step to produce the resin acids that required the addition of trimethylsilane (or is it Trimethylsilyl chloride). This is to ensure that the resin acids were detected (?).
Katrin was really helpful and showed Daisy and Jacob the derivization step.
After we got our first round of results, our ISO results were rather odd. Katrin was really nice and went through our results with us. She helped explain what the various peaks were and how to interpret the data and how to use the program. Katrin suggested Daisy and Marianne redo the experiments. Marianne recultured the yeast but put them in -Leu. These yeast are not -leu marked. The cultures did not grow the next day.
Another 5mL yeast culture was started. We need to transfer to 50 mL of SD media.
However, because we lacked some of the proper media, the yeast were put to SD -His -Leu media. The yeast are not -Leu marked. The yeast did not grow.
Now, another 5ml culture was started by Joe or Jacob.
The yeast were accidentally put in -His and -Leu media. However, Joe and Jacob only put it in for an hour before they realized it. The yeast were spun down and the incorrect media was poured out. Joe and Jacob were looking for SD -His media but it was Sunday and Alina was not there. Instead, they grew the yeast in YPD.
Then they switched it back to SG -His.
Daisy went to talk to Katrin and Anne the next day. Daisy explained what went wrong with the yeast. We looked at the yeast cultures and the ones without the CYP720B4 were growing fairly dense. Katrin and Anne were quite shocked at what we put the yeast through.
By putting the yeast into YPD, we are removing the selective marker. This means that the yeast can choose to get rid of the plasmid that it once needed when it was in the selective media.
Katrin then asked Daisy why it was not grown in -His and -Ura. Daisy was quite confused until she realized that the CYP720B4 was on a seperate vector that was -URA marked.
Because the entire time the yeast were not grown on -URA, it is possible that some yeast got rid of the plasmid that contained the CYP720B4 enzyme. This means that there would be less cells that would be able to produce the resin acids.
Daisy and Jacob extracted the samples. However, there was some odd contamination in the LAS sample. It appeared to be agar or some form of agarose gel.
When Daisy reported this to Katrin and Anne, both were again flabbergasted and quite shocked!
Daisy and Jacob decided not to run the LAS sample, which contained the contamination.
The samples were prepared.
Katrin helped run our samples. However Joe also wanted to run his monoterpene samples. However, this required a different program. However, none of us knew what the program was because Lina usually does it, but Lina is on vacation. Joe went to ask Chris and Chris says that we need to look at how Lina did it.
Katrin and Daisy and Joe went to go look for the program. Most of the programs were run on a DBWAX column. There was one protocol that ran on a Solgel column. However, someone was using it at the time.
Note: at this point, it is around 6pm.
Katrin and Anne try to add on the sample and do some crazy magic but somehow it does not work. It takes us another hour before we can get Joe's cineole samples to start running.
At this point, everyone is extremely tired and probably needs a coffee.
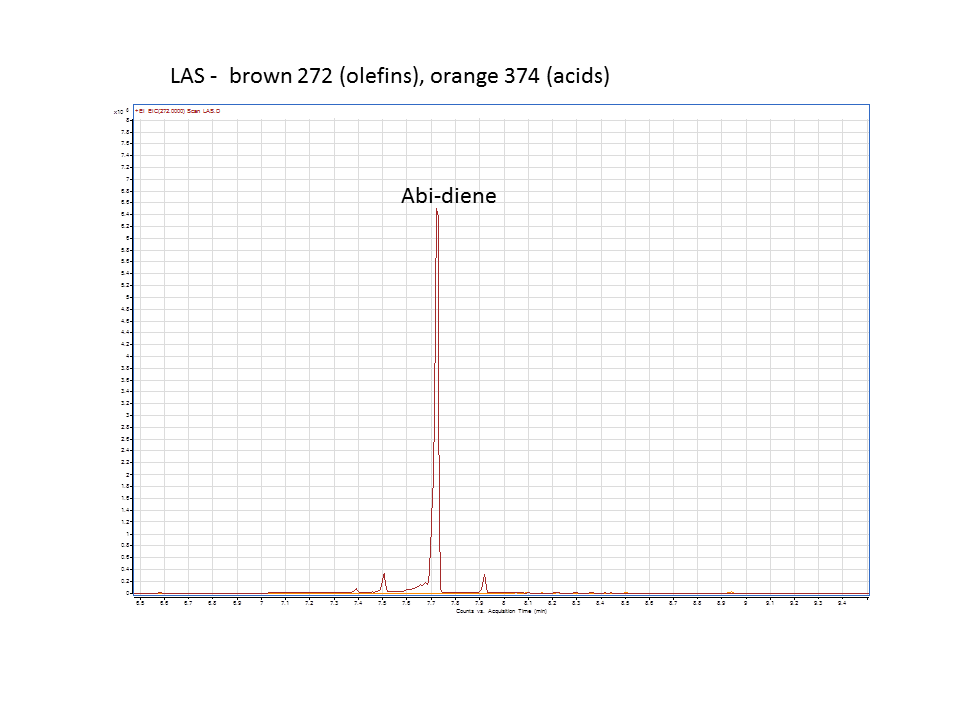
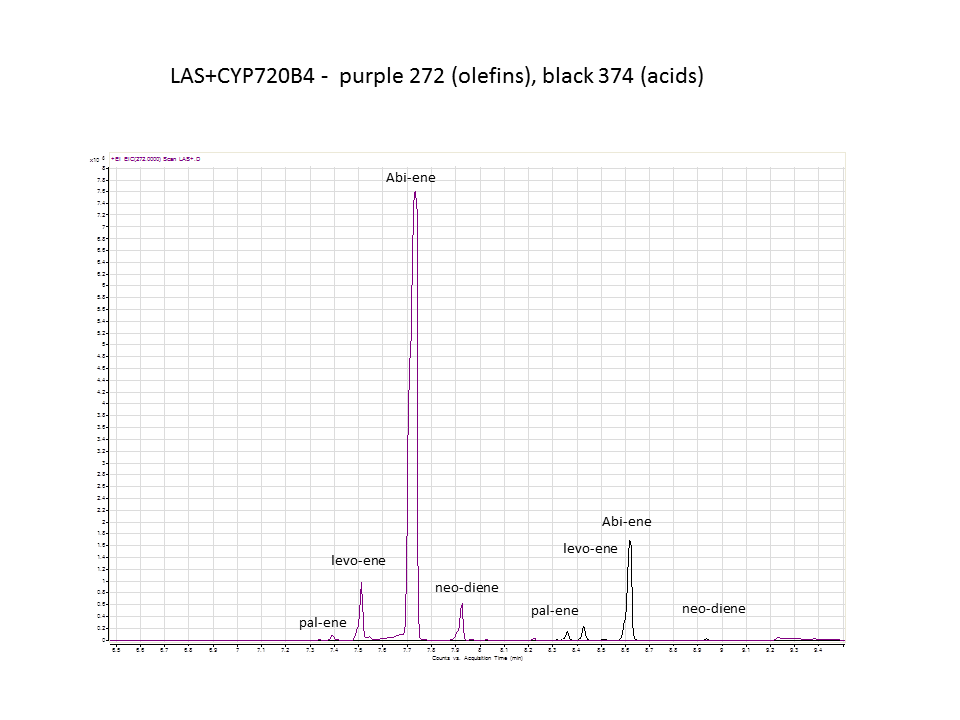
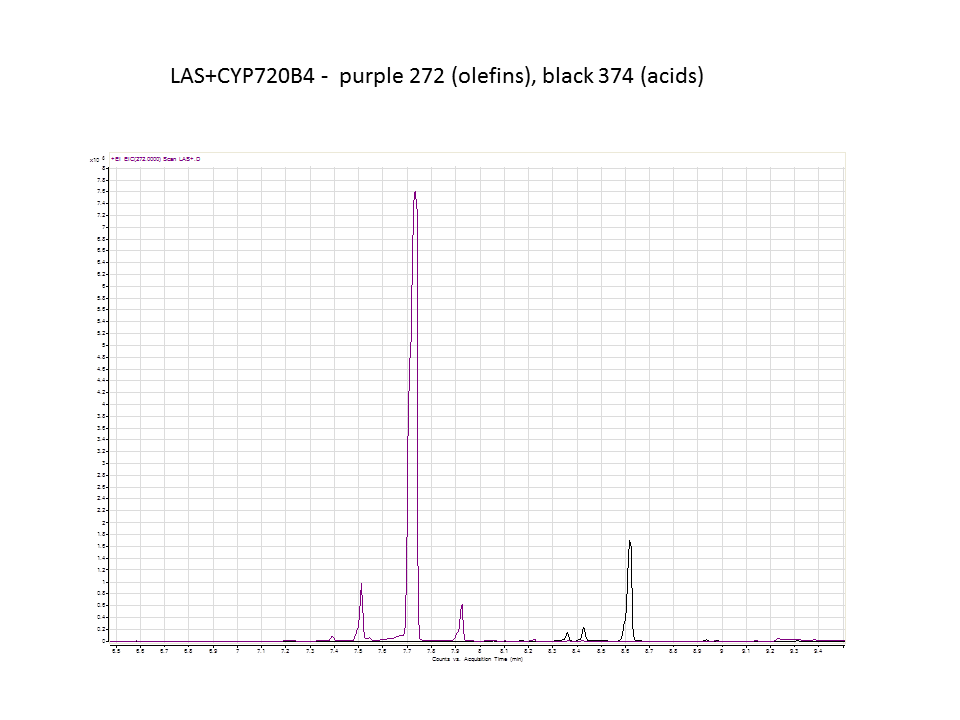
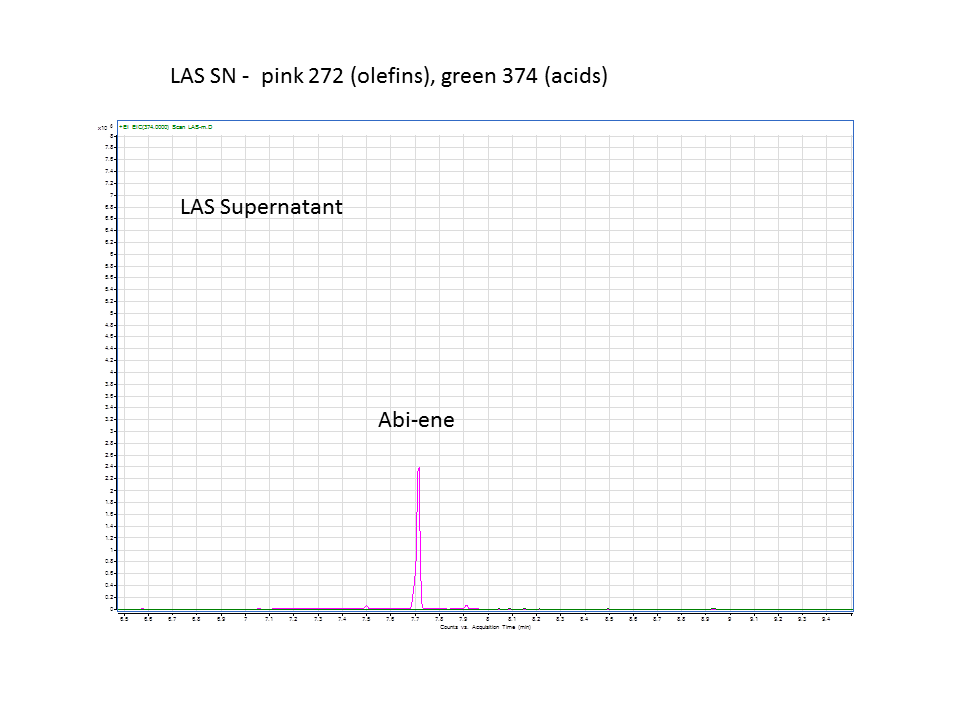
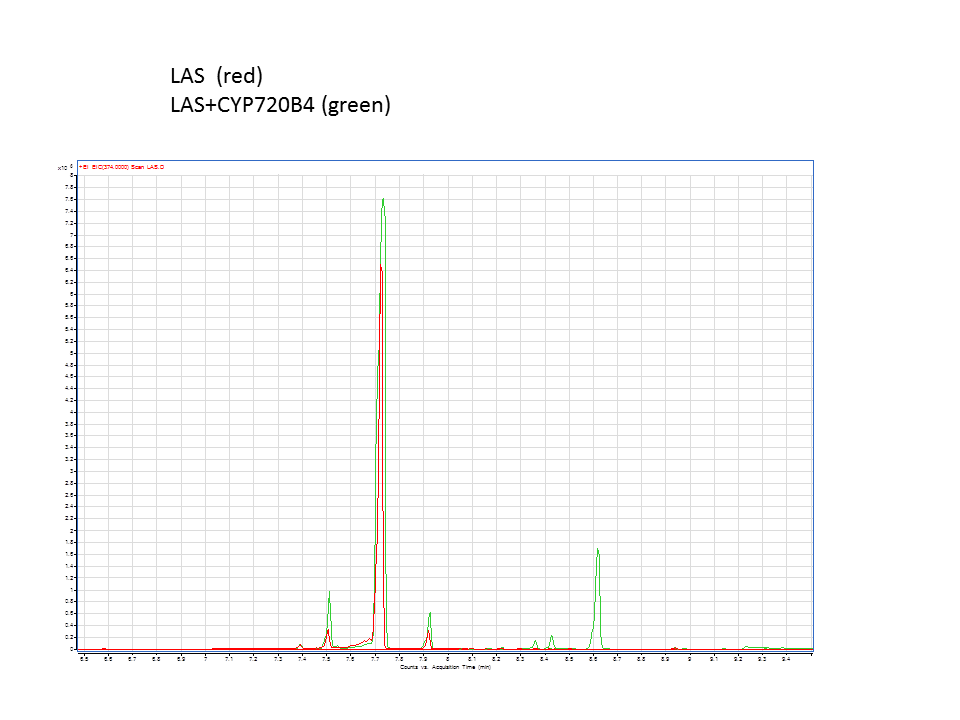
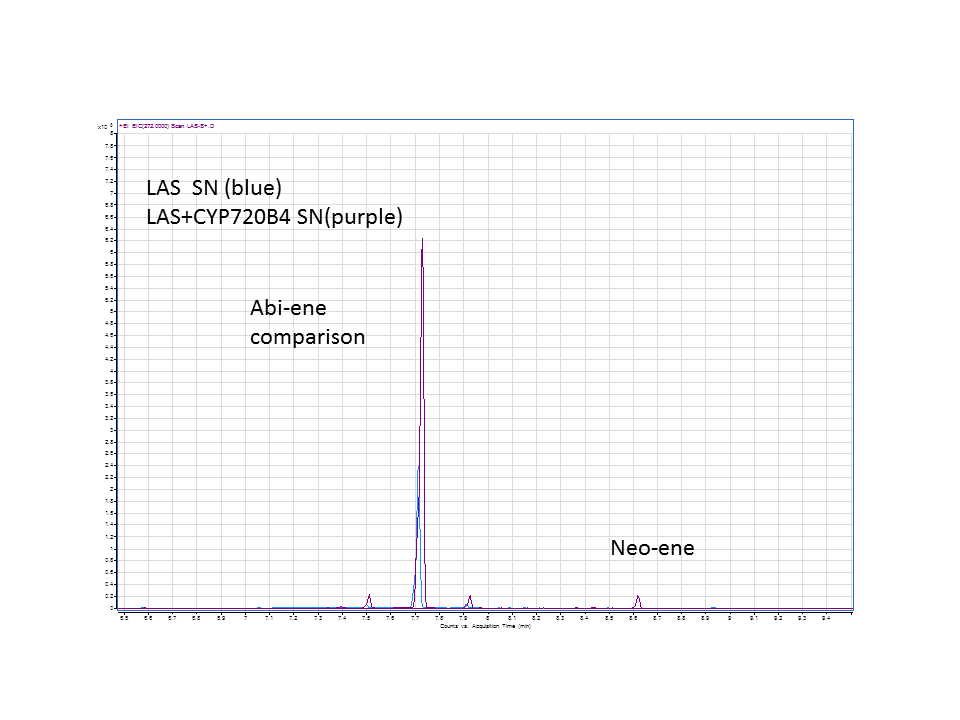
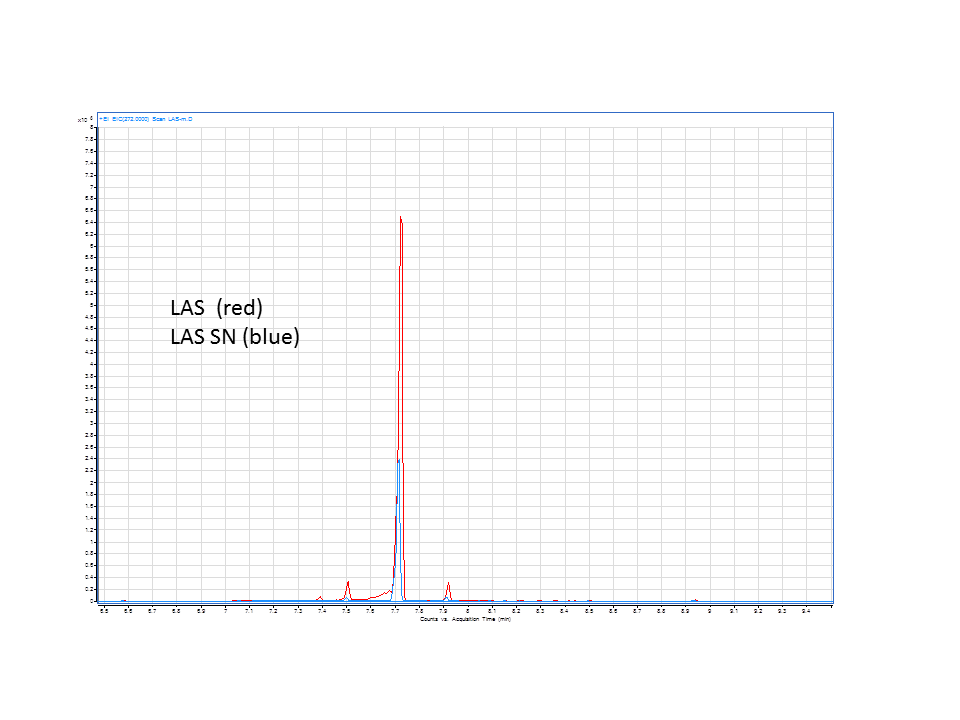
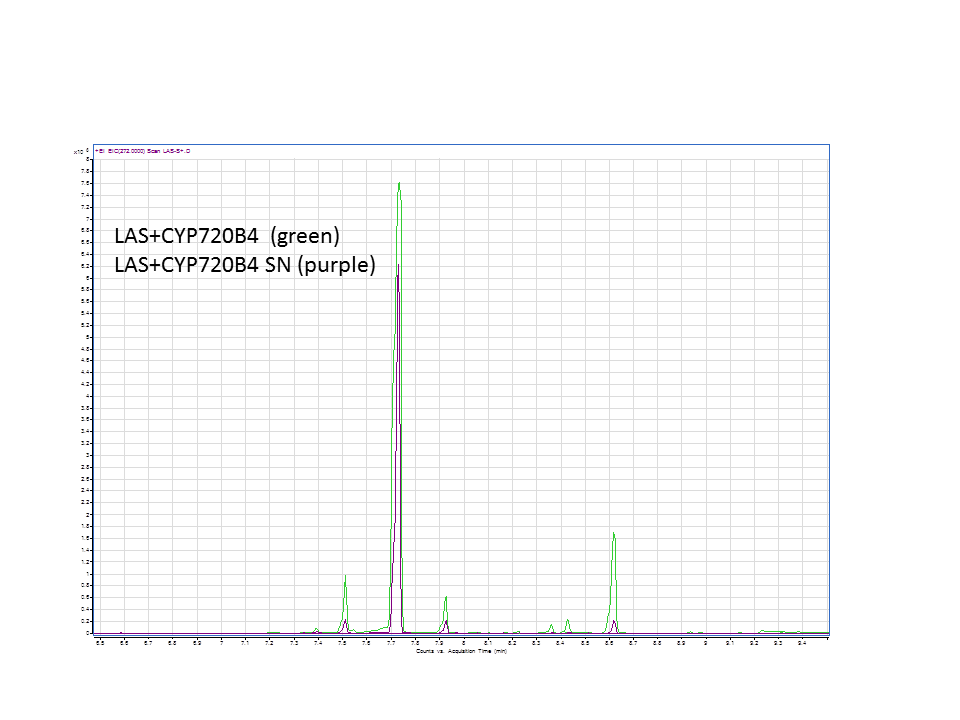
Katrin and Daisy now go and analyze the data from the latest diterpene results. The results were very interesting! Katrin did a very good job explaining to Daisy what the peaks were and how the results should be interpreted. Katrin also explained a lot of what she thinks the results may mean.
There was not any diterpenes produced from the ISO. LAS did produce diterpenes.
Katrin showed Daisy how to make the data and how to analyze it.
Katrin and Daisy finished at 9pm
These are the results from both runs.
Mountain Pine Beetle and Saccharomyces cerevisiae Co-culturing
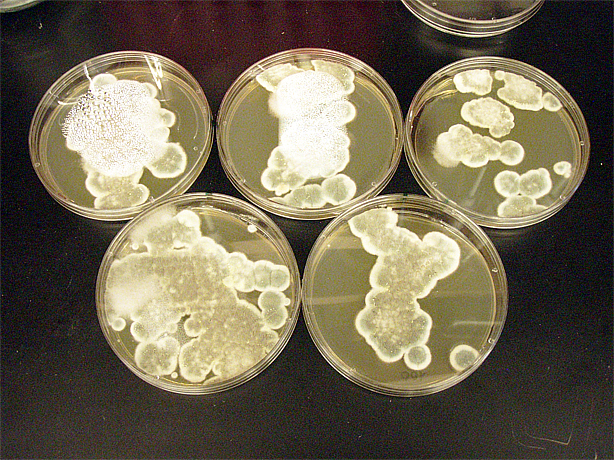
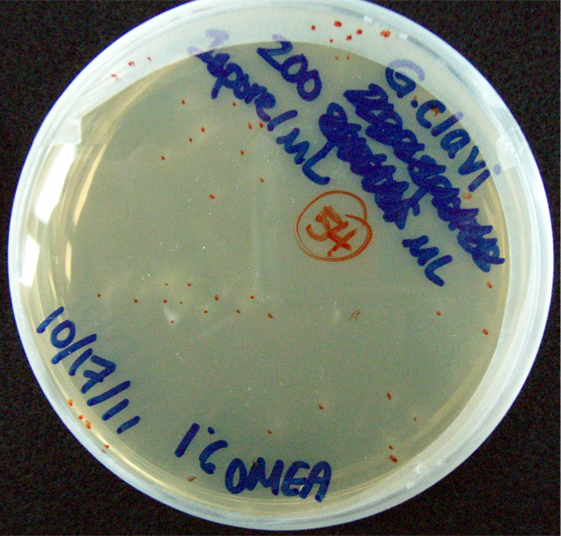
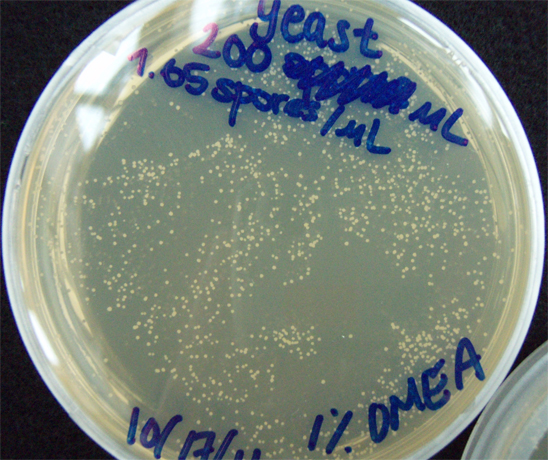
G. clavigera and S. cerevisiae co-culture experiments
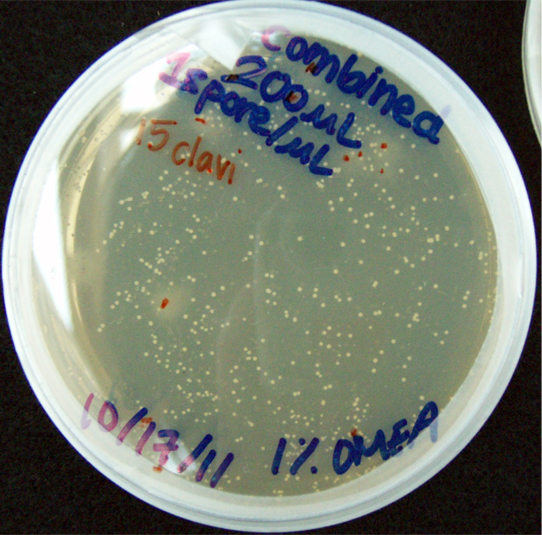
200 G. clavigera spores plated on 1% OMEA for 4 days.
330 S. cerevisiae spores plated on 1% OMEA for 4 days.
200 G. clavigera + 330 S. cerevisiae spores + plated on 1% OMEA for 4 days.
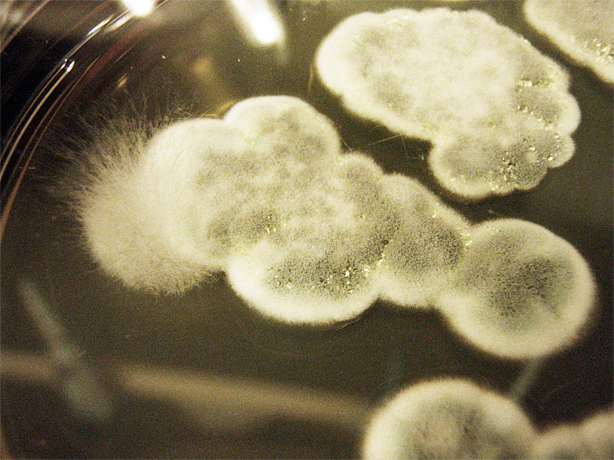
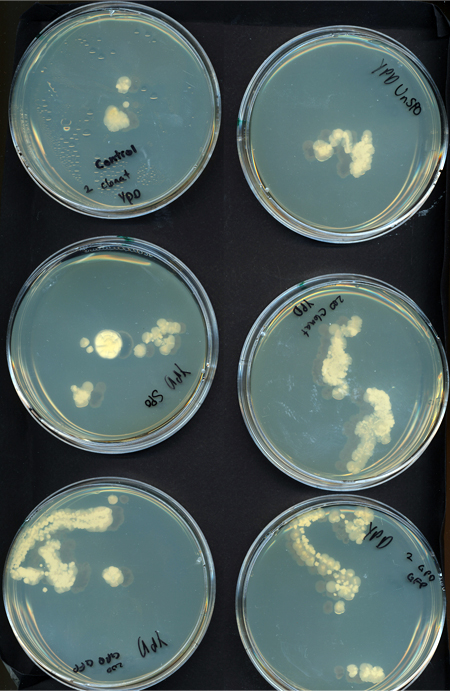
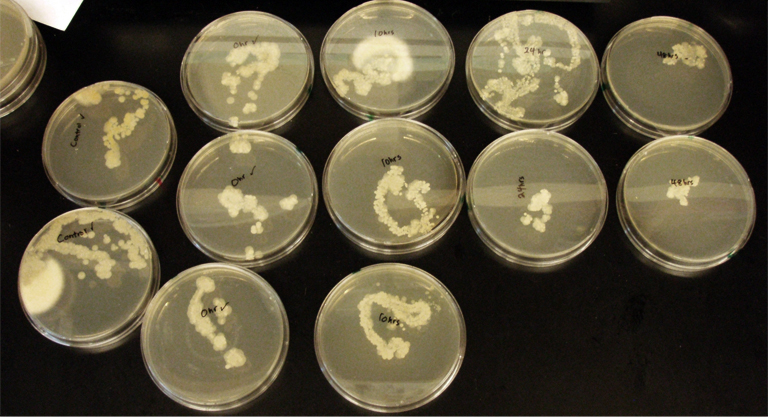
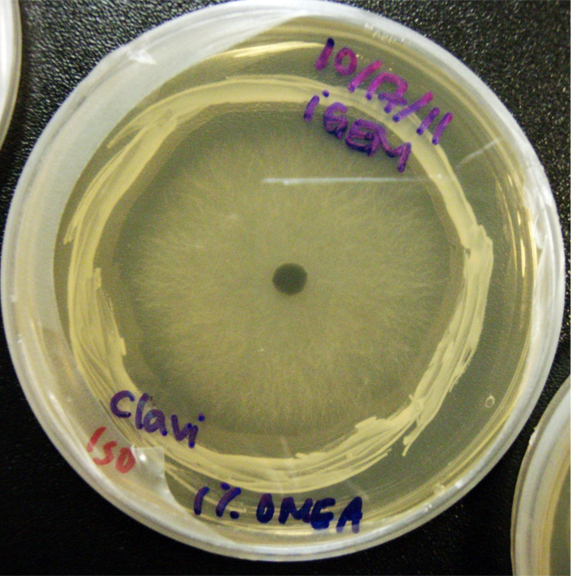
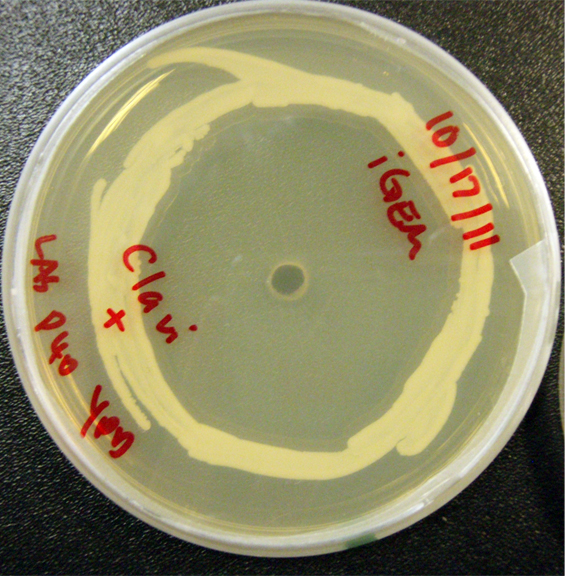
Assorted plates of G. clavigera mycelium plugs and terpenoid-producing S. cerevisiae rings.
S. cerevisiae grows well on YPD media but the G.claviegera grows very slowly.
S. cerevisiae grows slower on 1% OMEA but the G.clavigera grows well.
G. clavigera mycelium growing outwards towards diterpenoid ISO-producing S. cerevisiae on 1% OMEA.
G. clavigera mycelium growing very slowly outwards towards diterpenoid P40-producing S. cerevisiae on YPD.
Synthetic Biology Gone Wild: Expert Opinions
To obtain expert opinions on releasing synthetic organisms in the wild and initiate discourse on this topic, our team interviewed many experts across numerous fields at UBC.
For the interview transcripts and some of the key take-home messages, please view Expert Opinions on Synthetic Biology Gone Wild!
 "
"