Team:British Columbia/Notebook/Week 6
From 2011.igem.org

 |
 |
 |
 |
 |
Contents |
Lab Meeting - July 11
Gurpal gave a briefing on his meeting with the biosafety advisor at UBC; they talked about biosafety in Canada, the role of the Michael Smith Safety committee. We can now complete the safety issues page on the wiki!
Last year's returning team members (Marianne and Vicki) shared their experiences with the Jamboree last year to get everyone excited about regionals! Marianne and Laura began looking for accommodation during our stay in Indianapolis, and everyone is trying to figure out different flight options.
Hoping to start the presentation early, the team was assigned homework: everyone needs to watch at least one of the top presentations from last year (ex. Slovenia, Peking, Bristol, TU-Delft, Imperial College London, Cambridge) to gauge the calibre of presentations. Next meeting, we will discuss the pros and cons, and what we like about the presentations, and what we want ours to potentially look like.
Ideas for the wiki template were discussed: Vicki and Gurpal are thinking of having a big tree on the main page with menu buttons on the tree, with a pine beetle being the cursor. Brainstorming will continue.
There was a minor mishap in the lab (don't put glass centrifuge tubes into a particular centrifuge machine!), but the issue was dealt with appropriately, and in the end, everyone was happy!
1,8-Cineole
After 5 SDMs, transformations and minipreps, Jacob should now have ECORI-less, SPEI-less, XBAI-less, PSTI-less pg-tps-cin and pgxe-tps-cin. The gel of the restriction digest has the correct number of bands. Sequencing is the next step to confirm this result.
beta-Pinene
All 3 transformation plates (P2SDM1, P2SDM2, P2SDM3) have colonies! Marianne set up overnight cultures, mini-prepped them and sent them for sequencing. Hopefully the SDM worked and the beta-pinene synthase gene no longer has illegal cut sites.
(-)-Limonene
SDMs have failed thus far. More troubleshooting: use less pfu as the glycerol content in the enzyme could alter the reaction - prepared 3 samples, each with 0.5uL pfu, 1ul pfu, and 2ul pfu, respectively. Also prepare a sample using 0.5 taq polymerase only. Also increased the annealing temperature and elongation time.
Again, gel verification shows no bands. More troubleshooting: use a different cycling condition.
Again, no bands. Something is amiss... Vicki might start pulling out her hairs soon.
IDI1, HMG2 metabolic genes
Yeast colonies were found for HMG2, p330 and Cin, but not IDI1. The yeast transformed with IDI1 did not grow on a URA- plate. This may be because the nutrient marker was mutated in the plasmid, but this would not affect PCR to amplify the gene out. As a precaution, IDI1 was re-amplified.
alpha-Pinene
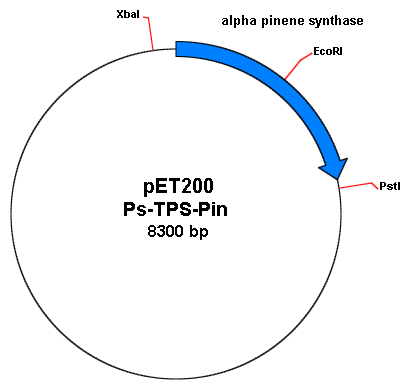
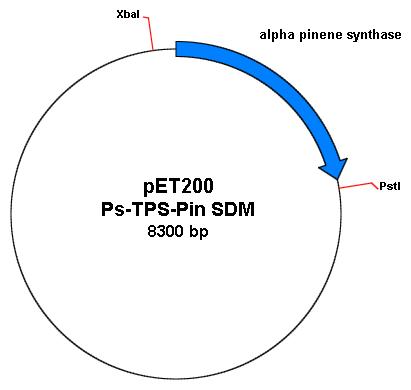
Joe performed a digest on the miniprepped product to see if his ECORI site is still present. He digested with EcoRI alone for both his SDMed product and his original plasmid. He also did another digest with XbaI, PstI (both of which are found on the pET200plasmid containing the alpha-synthase gene) and EcoRI. To visualize, here are the plasmid maps of the pET200 containing the alpha-pinene synthase gene.
(Note: plamid map distance is not to scale. XbaI to PstI is around 3.4kb; the alpha pinene synthase (Ps-TPS-pin) is about 1.8kb with its EcoRI site at 960bp away from the start of the gene.)
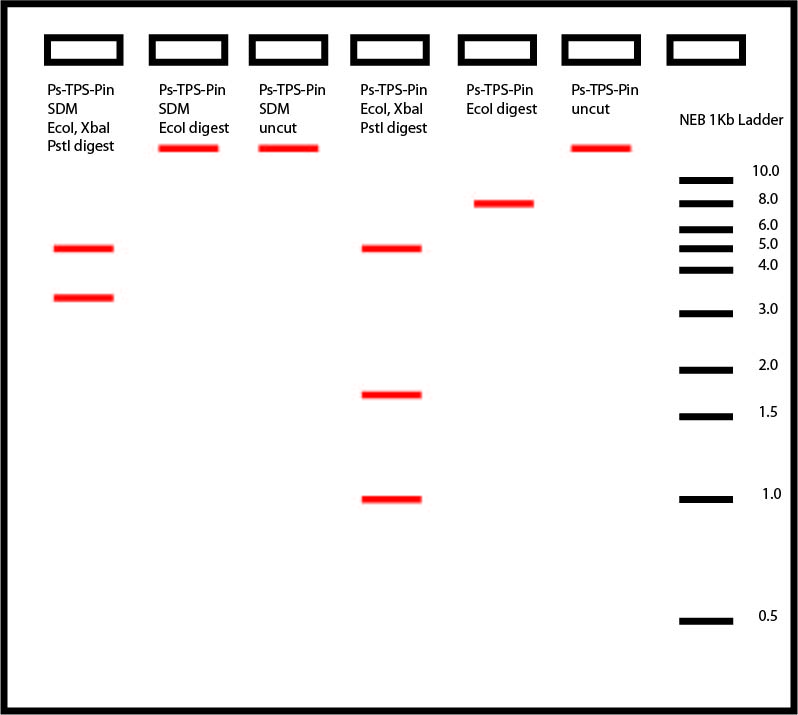
Therefore, after gel electrophoresis, the following results should be observed, which were observed in Joe's gel:
The uncut plasmids, should run slower than cut plasmids on the gel. Here, it runs slightly above the 1kb ladder. The EcoRI cut on the original pET200 with Ps-TPS-Pin shows up as a band at around 8kb long. Conversely, the EcoRI cut on the SDM pET 200 with Ps-TPS-Pin, shows a similar band as uncut pET200-Ps-TPS-PinSDM. This shows that the site directed mutagenesis worked and my EcoRI site is removed.
To be entirely sure, I also digested with XbaI, PstI, and EcoRI on both the SDM pET200 Ps-TPS-Pin and original pET200Ps-TPS-Pin. 3 expected bands (4.9kb, 1.8kb, and 0.6kb) showed up from cut original pET 200Ps-TPS-Pin, while only 2 expected bands (4.9kb and 3.4kb) showed up from cut SDM pET200 Ps-TPS-Pin.
 "
"