Team:SJTU-BioX-Shanghai/Project/Subproject3
From 2011.igem.org
(Difference between revisions)
(→Results) |
(→Results) |
||
| Line 44: | Line 44: | ||
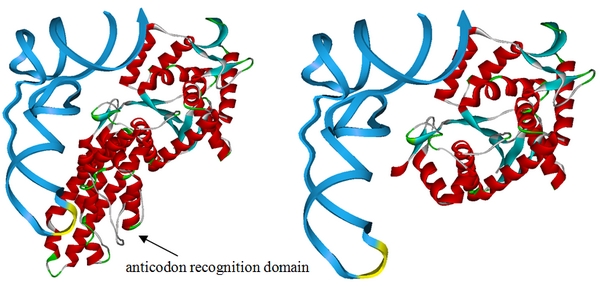
| - | [[image:11SJTU-MetRS.jpg|frame|center|''Fig | + | [[image:11SJTU-MetRS.jpg|frame|center|''Fig.1'' This design is based on the crystal structure of methionyl-tRNA synthetase complex with tRNA (PDB ID:2CSX). We have superimposed the crystal structure of methionyl-tRNA synthetase from ''E.coli'' and obtained the overlay structure after kinetics optimization. Above is the picture showing ''E.coli'' methionyl-tRNA synthetase with (left) and without (right) anticodon recognition domain. The picture proposed that ''E.coli'' methionyl-tRNA synthetase will lose the ability to bind tRNA<sup>Met</sup> anticodon if anticodon recognition domain is deleted, thus losing anticodon specificity while maintaining aminoacylation ability. '''We have built the truncated MetRS, PT7-''metG''N ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567015 BBa_K567015]), based on this design. ''']] |
| Line 53: | Line 53: | ||
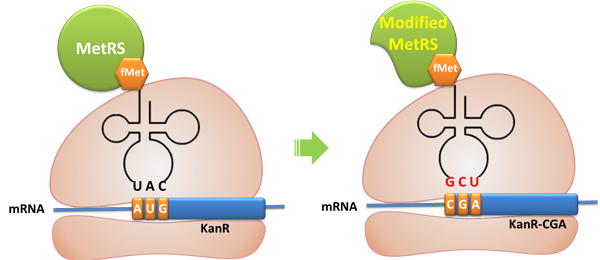
We screened the MetRS obtained through error-prone PCR and obtained one target mutant. We have also tested the activity of the truncated MetRS PT7-''metG''N ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567015 BBa_K567015])and found that the truncated MetRS acted as expected, losing specificity for tRNA<sup>Met</sup> anticodon while maintaining aminoacylation ability. | We screened the MetRS obtained through error-prone PCR and obtained one target mutant. We have also tested the activity of the truncated MetRS PT7-''metG''N ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567015 BBa_K567015])and found that the truncated MetRS acted as expected, losing specificity for tRNA<sup>Met</sup> anticodon while maintaining aminoacylation ability. | ||
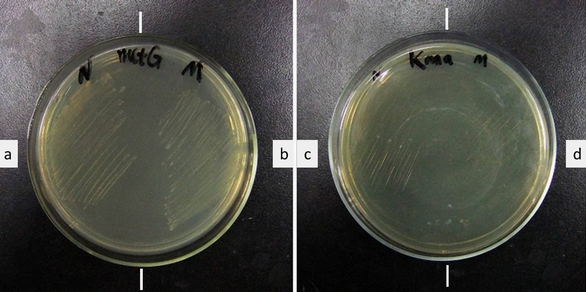
| - | [[image:11SJTU-initial_codon_result.jpg|frame|center|''Fig | + | [[image:11SJTU-initial_codon_result.jpg|frame|center|''Fig.2'' Growth of ER2566 with a. ''metG''N + ''metY''-CGA, b. ''metG''M + ''metY''-CGA, c. + ''metG''N, d. + ''metG''M. Growth medium (left): LB Kana+Tet. Growth medium (right): LB Kanamycin.'''Cells survived Kanamycin with our device. Without our device, cells cannot survive.''' ]] |
Cell growth shows that the cells have Kanamycin resistance only when both modified MetRS (''metG''N or ''metG''M) and modified tRNA<sup>Met</sup>(''metY''-CGA) are transformed into the cell, '''proving that tRNA ''metY''-CGA can transfer fMet to CGA when it is used as the start codon and that ''metG''N and ''metG''M work well. ''' | Cell growth shows that the cells have Kanamycin resistance only when both modified MetRS (''metG''N or ''metG''M) and modified tRNA<sup>Met</sup>(''metY''-CGA) are transformed into the cell, '''proving that tRNA ''metY''-CGA can transfer fMet to CGA when it is used as the start codon and that ''metG''N and ''metG''M work well. ''' | ||
Latest revision as of 00:42, 29 October 2011
 "
"