|
Initial-Codon Switch
Introduction
The Rare-Codon Switch can only regulate the amount of target protein. To make our device a strict molecular switch that turns on/off protein biosynthesis, we need to eliminate the background.
Protein biosynthesis begins when initial tRNAfMet brings fMet to the initial codon.
Design
We design an Initial-Codon Switch to turn on/off protein translation.
We can control the translation process by controlling whether the ribosome can get through the changed initial codon placed in the target protein's mRNA. In E.coli, the original initial codon is AUG. If the initial codon is changed to any other codon, translation cannot be initiated. Based on this, a strict switch can be achieved by controlling the existence of charged tRNAfMet-TCG base pairing codon CGA. This process is controlled by two elements:
- The existence of tRNAfMet-TCG
- MetRS that can charge tRNAfMet-TCG
- KanR-CGA: the initial codon of KanR gene is changed from AUG to CGA.
Results
In order to charge Met to tRNAMet with mutated anticodon, we need to deprive MetRS of its anticodon specificity. Two strategies are used:
- Directed Evolution Strategy: error-prone PCR is used to introduce random mutations into MetRS.
- Rational design of MetRS: we have obtained a truncated MetRS without anticodon recognition domain. The structure of the truncated protein is shown below. This modified MetRS can charge tRNAMet without recognizing its anticodon.
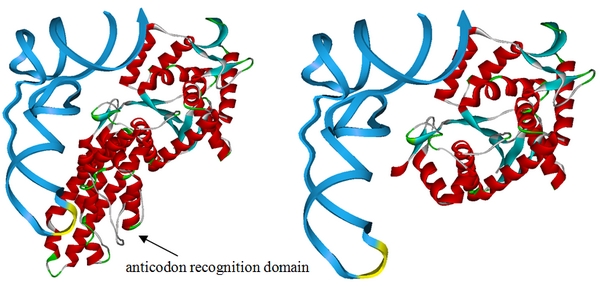 Fig.1 This design is based on the crystal structure of methionyl-tRNA synthetase complex with tRNA (PDB ID:2CSX). We have superimposed the crystal structure of methionyl-tRNA synthetase from E.coli and obtained the overlay structure after kinetics optimization. Above is the picture showing E.coli methionyl-tRNA synthetase with (left) and without (right) anticodon recognition domain. The picture proposed that E.coli methionyl-tRNA synthetase will lose the ability to bind tRNAMet anticodon if anticodon recognition domain is deleted, thus losing anticodon specificity while maintaining aminoacylation ability. We have built the truncated MetRS, PT7-metGN ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567015 BBa_K567015]), based on this design.
metY-CGA: We have cloned operon metY containing tRNAMet from E.coli to pACYC184. The anticodon of tRNAMet was mutated to TCG (base pairing codon CGA).
We inserted bla operon from pUC18 into pET28a, endowing pET28a with Kan and Amp resistance. Then the start codon of KanR gene is mutated from ATG to CGA. We then inserted MetRS obtained from error-prone PCR into the MCS of this pET28a. When this plasmid is co-transformed with metY-CGA, we can screen the mutants with Kanamycin resistance. The same method is also used to test the function of the truncated MetRS PT7-metGN ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567015 BBa_K567015]).
We screened the MetRS obtained through error-prone PCR and obtained one target mutant. We have also tested the activity of the truncated MetRS PT7-metGN ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567015 BBa_K567015])and found that the truncated MetRS acted as expected, losing specificity for tRNAMet anticodon while maintaining aminoacylation ability.
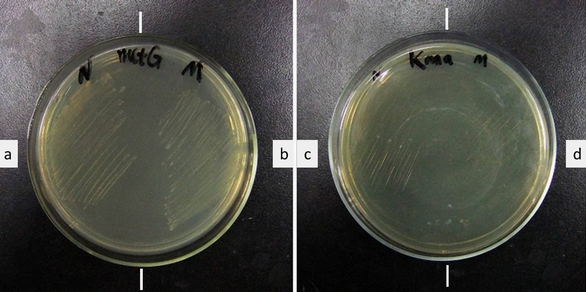 Fig.2 Growth of ER2566 with a. metGN + metY-CGA, b. metGM + metY-CGA, c. + metGN, d. + metGM. Growth medium (left): LB Kana+Tet. Growth medium (right): LB Kanamycin.Cells survived Kanamycin with our device. Without our device, cells cannot survive. Cell growth shows that the cells have Kanamycin resistance only when both modified MetRS (metGN or metGM) and modified tRNAMet(metY-CGA) are transformed into the cell, proving that tRNA metY-CGA can transfer fMet to CGA when it is used as the start codon and that metGN and metGM work well.
Initial-Codon Switch proved successful.
Conclusions
We have successfully constructed the Initial-Codon Switch, including the modified MetRS (metGN or metGM) and modified tRNAMet(metY-CGA) as well as the Reporter KanR. We have tested the device and results proved Initial-Codon Switch as a strict molecular switch without background noise. metY-CGA can transfer fMet to CGA when it is used as the start codon and that metGN and metGM is able to charge metY-CGA with Met.
|