Team:SJTU-BioX-Shanghai/Project/Subproject1-2
From 2011.igem.org
|
|
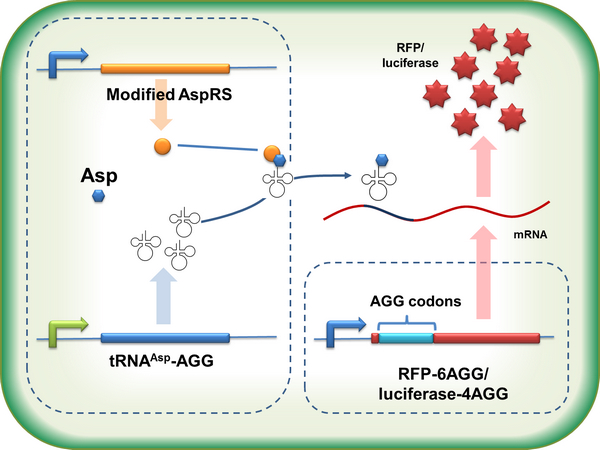
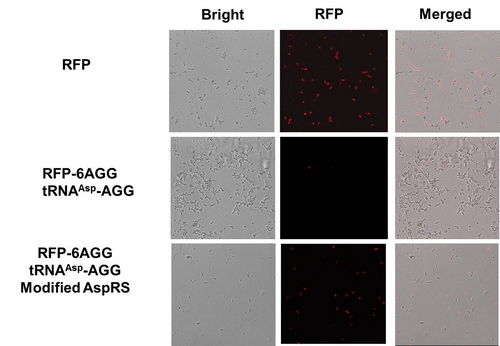
aaRSDesignWe control the translation process by modifying the tRNA and aaRS that are originally not for Arg. tRNAAsp-AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567012 BBa_K567012]) : tRNAAsp with its anticodon mutated to CCU can base pair with rare codon AGG, which is originally the codon for Arg. This tRNA is under the constitutive aspV promoter. TDRS ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567011 BBa_K567011]) : Aspartyl aminoacyl tRNA synthetase (AspRS) without anticodon recognition domain under the control of T7 promoter and lac operator. To deprive AspRS of its anticodon specificity, we analyzed the structure of AspRS and expressed a truncated AspRS without anticodon recognition domain. This modified enzyme keeps its ability of aminoacylation while loses its activity of recognizing anticodon of tRNA. Reporter:We test our design with two reporters. RFP-6AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567017 BBa_K567017]) : RFP with 6AGG insertions Luciferase-4AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567009 BBa_K567009]) : luciferase with 4AGG insertions ActionWhen the modified enzyme is produced under induction, it can charge tRNAAsp-AGG with Arg. Then the charged tRNA can get through the rare codons on the mRNA, so that RFP or luciferase can be produced. ResultWe have used PT7-RFP-6AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567017 BBa_K567017]) as our Reporter. We have constructed tRNAAsp-AGG and PT7-TDRS (AspRS without anticodon recognition domain) ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567012 BBa_K567012] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K567011 BBa_K567011]). tRNAAsp-AGG, which can recognize rare codon AGG, is under constitutive promoter. With our device, RFP is successfully produced. Without our device, little RFP is observed. 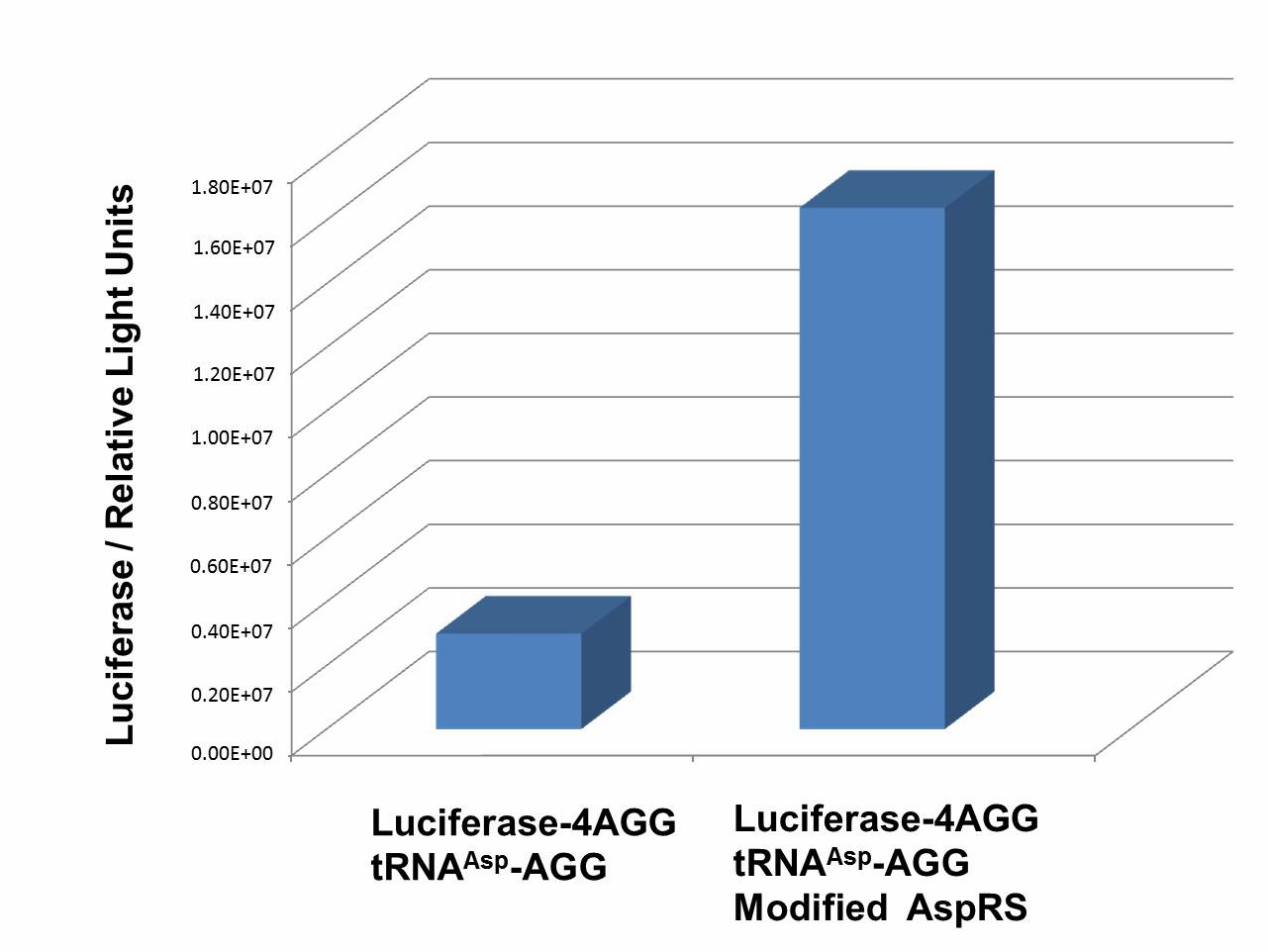 Fig.3 Examination of luciferase production with and without device. ER2566 cannot produce luciferase with PT7-Luc-4AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567009 BBa_K567009]) only. When tRNAAsp-AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567012 BBa_K567012]) and PT7-TDRS ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567011 BBa_K567011]) are co-transformed into the cell, luciferase production is increased. The results proved that aaRS can regulate protein biosynthesis. We have used PT7-Luc-4AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567009 BBa_K567009]) as our Reporter to test the function of PT7-TDRS ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567011 BBa_K567011]) and tRNAAsp-AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567012 BBa_K567012]). Results are shown above. Luciferase production has been largely increased with our device. The function of Switch is characterized by the amount of luciferase expressed. The amount of luciferase expressed is reflected by the light emitted when luciferase acts on the appropriate luciferin substrate. The light can be measured by luminometer and the quantity is positively correlated with the amount of luciferase and its activity (learn more...). We successfully controlled protein expression by manipulating aaRS.
|
 "
"