Team:British Columbia/Model1
From 2011.igem.org

MODEL 1: Secretion and Production of Monoterpenes in Yeast
OBJECTIVE: To create a model to assay the production of monoterpenes in our genetically engineered yeast cells given that they contain the metabolic genes IDI1, K6R-hmg2, erg20-2 and a synthase gene (one of PgTPS-Pin2, PsTPS-Car1, PgxeTPS-Cin, PgTPS-Cin and PsTPS-Pin). Model Creator: Gurpal BisraFirst, a series of block diagrams to model the biochemical pathways were created. To illustrate, I started with the mevalonate pathway (Ignea et al. 2011) and simplified the pathway to only relevant enzymes associated with our project – the IDI1, HMG2 and ERG20. Since each yeast cell contained a different synthase gene, the end of each biochemical pathway was modified accordingly to produce to correct monoterpenes (Keeling et al. 2011). Next, a series of chemical equations were created. For instance, 6 chemical reactions are common to each yeast cells. This part was important because I began to note the kinetic constants. Afterwards, I created differential equations based on the simplified biochemical pathways and chemical reactions. Dr. Eric Lagally referred me to 2 sources to help me make this conversion: “Writing Differential Equations” (Bourne 2010) and “Chapter 6- Transport and Kinetics” (Jakubowski 2011). The differential equations I made were based on using first order and Michelson-menten kinetics. At this point, many constants and concentrations must be known to solve the differential equations. In particular, there is an enzyme database called “BRENDA” which contains some of the values – kcat and km (Scheer 2011). Our project is based on research that has been conducted in 2011 so some variables will need to be experimentally determined. The solutions to these differential equations will be solved in MATLAB and plots of concentration of monterpene as a function of substrate or time will be created.
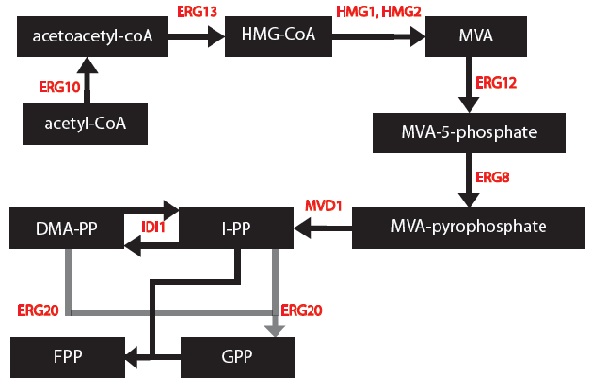
Source*: Figure 1 from "Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids."
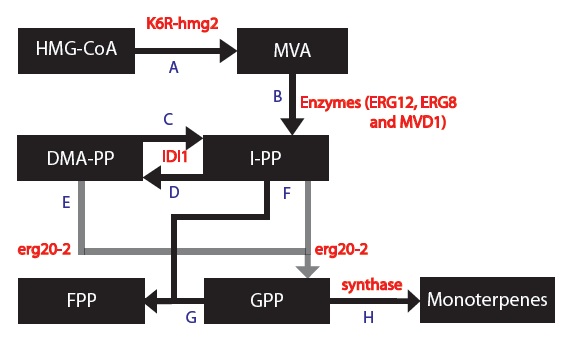
Now, our group wanted to mass produce monoterpenes so we focused on the metabolic enzymes: 1) Replace HMG2 with K6R-hmg2
why? "Mutation of the lysine 6 into arginine in HMG2 is known to stabilize the protein from protein from protein degradation**."
Source*: "Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids" under - Integration of K6R variant of HMG2 into the genome. Source**: Gardner RG, Hampton RY: A 'distributed degron' allows regulated entry into the ER degradation pathway. Embo J 1999, 18(21): 5994-6004.
2) Replace ERG20 with erg20-2
why? ergo20-2 is a mutant of the ERG20 gene with a K197 to E substitution. The encoded mutant gene produces "25% FPP and 75% GPP instead of 75% FPP and 25% GPP" as compared to the wildtype ERG20 gene. We want more GPP than FPP because our goal is to produce monoterpenes and not diterpenoids.
Source*: "Monoterpenoid biosynthesis in Saccharomyces cerevisiae." Source*: "Pathway engineering for functional isoprenoids."
3) Introduce a gene which encodes for a synthase to combine with GPP to form monoterpenes. We chose 5 different synthases that each produces different monoterpenes in different proportions.
Source: "Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea spp.)."
Next, we can now form a modified and simplified model for the Mevalonate Pathway as shown below,
At this point, it is important to note 2 things: 1) The enzymes ERG12, ERG8 and MVD1 are NOT RATE LIMITING; therefore, we chose not to include them in our simplified model. However, these enzymes are accounted for in our simulations.
2) The RATE LIMITING STEP IS WITH THE HMG2 enzyme.
Source: http://www.yeastgenome.org/cgi-bin/locus.fpl?locus=hmg2
Next, we are using 5 different synthases which each produce monoterpenes. Tests have already been performed in vivo in test tubes (no organisms) to determine the following information:
Synthase PgTPS-Pin2 catalyzes GPP into producing 70.5% β-pinene and 29% α-pinene + other monoterpens in negligible amounts.
Synthase PsTPS-Car1 catalyzes GPP into producing 66.4% 3-carene and 2.7% α-pinene + other monoterpens in negligible amounts.
Synthase PgxeTPS-Cin catalyzes GPP into producing 65.6% 1,8 cineole, 3.0% α-pinene and 2.6% β-pinene + other monoterpens in negligible amounts.
Synthase PgTPS-Cin catalyzes GPP into producing 81.1% 1,8 cineole, 1.9% α-pinene and 1.9% β-pinene + other monoterpens in negligible amounts.
Synthase PsTPS-Pin catalyzes GPP into producing 83.4% α-pinene and 12.6% β-pinene + other monoterpens in negligible amounts.
Source*: "Transcriptome mining, functional characterization, and phylogeny of large terpene synthase gene family in spruce (Picea spp.)" under Table 2: Product files of recombinant TPS enzymes based upon total ion current of GC-MS analysis of DB-WAX column.
I made a list of important common chemical reactions after developing this simplified version of the biochemical pathway:
Since each synthase gene combines with GPP to produce different monoterpenes, some chemical reactions will be specific to a certain synthase.
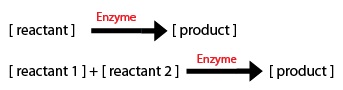
Now, I needed to model these chemical equations as a series of differential equations. I don't have a biochemistry background (I am an engineer) so this problem was new for me. By looking at the website: http://www.boomer.org/c/p3/c02/c0207.html , I learned the basics to get started. 2 things to take into account are: 1) The direction of the arrow: -if the arrow goes into a component, the equation segment is positive -if the arrow leaves a component, the equation segment is negative
2) Types of Rate Processes -if the rate process is zeroth order, just enter the rate constant -if the rate process i first order, multiply the rate constant by the initial substrate concentration -for a Michaelis-Menten process, include the amount or concentration of the component at the tail of the arrow (call it X1 for example) and you will get: d(X1)/dt = (-Vm*X1)/(Km+X1)
I noticed that each step is based on an enzyme so I decided to use Michaelis-Menten kinetics to model the simplified biochemical pathway.
Most of the equations fall under the main 2 types of Michaelis–Menten equations:
For the first type of reaction, please look at this website: http://employees.csbsju.edu/hjakubowski/classes/ch331/transkinetics/o...). I had to make 2 assumptions to use the equation:
1) At steady state, [ES] does not change with time, therefore it's derivative with time is = 0. Therefore, k1[E][S] = (k2+k3)[ES]
2) S >> Eo
Next, the second type of Michaelis-Menten kinetic equation I needed was one for the case where I have 2 substrates combining into 1 product via a condensation reaction. In fact, "DMA-PP and I-PP are condensed to generate geranyl diphosphate (GPP, C10), which is further converted with IPP into ferensyl diphosphate (FPP, C15), with prenyl transferase such as FPP synthase."
Source*: "Pathway engineering for functional isoprenoids."
For a visual representation, below is the 2 step reaction which shows the catalyzation via FPP synthase,
Source: "The role of ERG20 gene (encoding yeast farnesyl diphosphate synthase) mutated in long dolichol formation. Molecular modeling of FPP synthase"
To model these types of reactions, I found an expression for the reaction velocity to be: v = d(X)/dt = (Vmax)/( (kms1/[S1]) + (kms2/[S2]) + 1 ) where [S1] = concentration of substrate 1, [S2] = concentration of substrate 2. They have an associated kinetic value. Vmax = kcat*[E] where [E] = the concentration of enzyme.
The final thing I needed to consider before writing out the differential equations were how to use the in vivo data indicating what percentage of monoterpenes were produced. These final steps also took the form of the Michaelis-Menten equations but I modified them by multiplying each by the fraction of product produced. For example, please look at equations 10 and 12 in the differential equations below to see fraction of products are produced for the PgTPS-Pin2 synthase.
Next, I created differential equations from the series of chemical reactions and block diagrams for each of the biochemical pathways.
These are the differential equations that are common to each biochemical pathway:
Since each synthase gene combines with GPP to produce different monoterpenes, some differential equations will be specific to a certain synthase.
The differential equations specific to the PgTPS-Pin2 synthase gene:
The differential equations specific to the PsTPS-Car1 synthase gene:
The differential equations specific to the PgxeTPS-Cin synthase gene:
The differential equations specific to the PgTPS-Cin synthase gene:
The differential equations specific to the PsTPS-Pin synthase gene:
 "
"