Team:SJTU-BioX-Shanghai/Project/Subproject1-3
From 2011.igem.org
(Difference between revisions)
(→Number of Rare Codons) |
|||
| Line 30: | Line 30: | ||
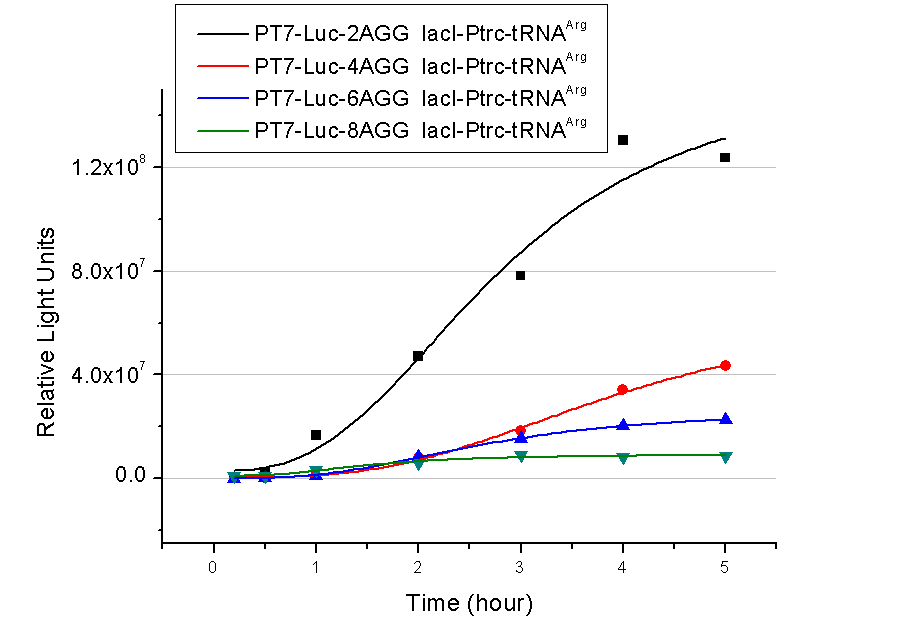
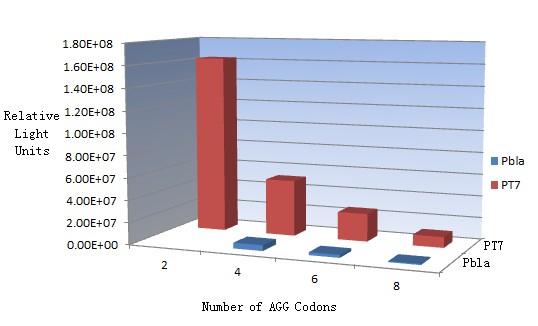
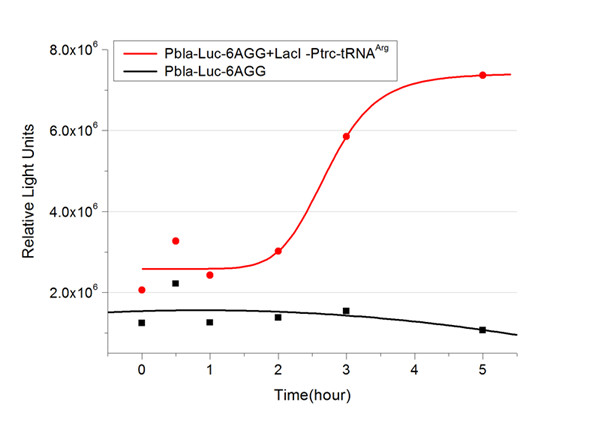
Results show that the more rare codons are inserted, the lower the background expression and the narrower the range our device can regulate. | Results show that the more rare codons are inserted, the lower the background expression and the narrower the range our device can regulate. | ||
| - | [[image:11SJTUzhuzhuangtu-compare.jpg|frame|center|''Fig. | + | [[image:11SJTUzhuzhuangtu-compare.jpg|frame|center|''Fig.1'' Enzyme activity of luciferase reaching plateau phase.]] |
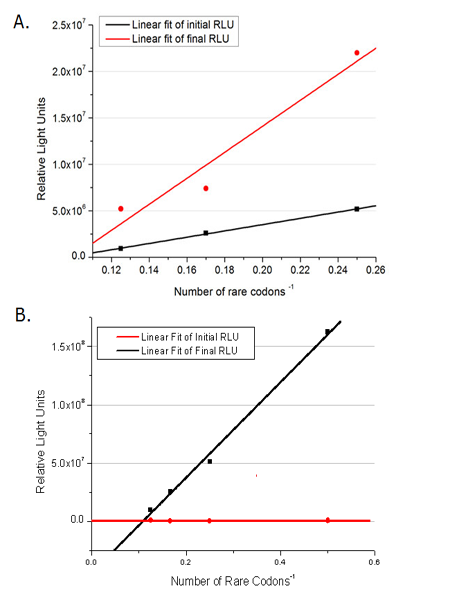
This picture reflects more clearly that the more rare codons are inserted, the lower the background expression and the narrower the range of device regulation. We are able to predict the outcome of influence of different number of rare codons in protein biosynthesis, offering valuable information for device usage. | This picture reflects more clearly that the more rare codons are inserted, the lower the background expression and the narrower the range of device regulation. We are able to predict the outcome of influence of different number of rare codons in protein biosynthesis, offering valuable information for device usage. | ||
| - | [[image:11sjtu_Compare_DATA_Fitting.png|frame|center|''Fig. | + | [[image:11sjtu_Compare_DATA_Fitting.png|frame|center|''Fig.2'' The comparison between background expression and induced expression of luciferase with different rare codon insertions. (A)PT7-luc reporters. (B) P''bla''-luc reporters]] |
===Influence of different strengths of target protein promoters=== | ===Influence of different strengths of target protein promoters=== | ||
| Line 40: | Line 40: | ||
We examined the influence of different Reporter promoters on the working curve of our device, which is reflected by luciferase activity. The working range of our device is pre-defined by the strength of target protein promoter, T7 promoter and ''bla'' promoter in our project. | We examined the influence of different Reporter promoters on the working curve of our device, which is reflected by luciferase activity. The working range of our device is pre-defined by the strength of target protein promoter, T7 promoter and ''bla'' promoter in our project. | ||
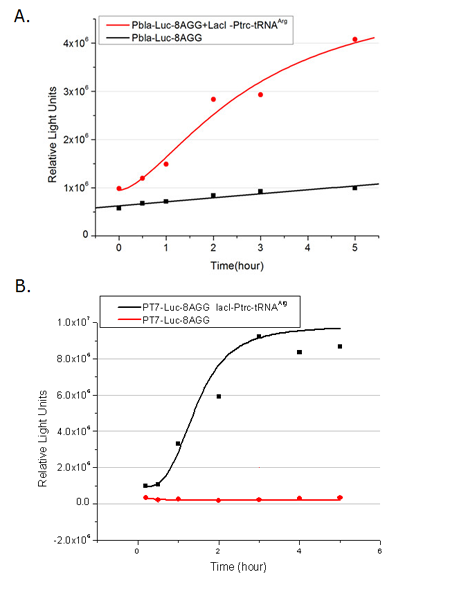
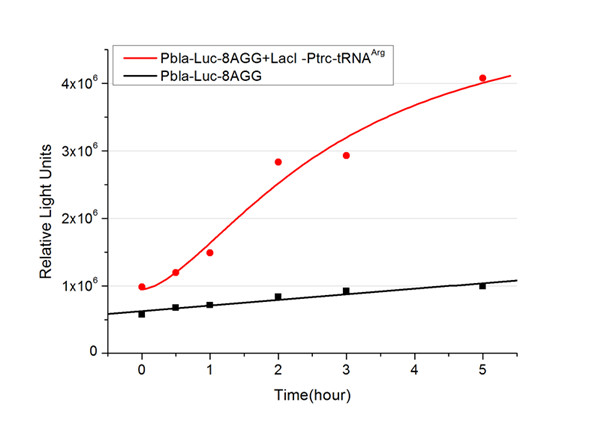
| - | [[image:11sjtu_Compare_8.png|frame|center|''Fig. | + | [[image:11sjtu_Compare_8.png|frame|center|''Fig.3'' (A) Working curve of tRNA Modulator ''lacI''-Ptrc-tRNA<sup>Arg</sup> under Reporter P''bla''-Luc-8AGG reflected by bioluminescence emitted from the luciferin reaction. (B) Working curve of tRNA Modulator under Reporter PT7-Luc-8AGG. Here we analyze the influences of strong/weak promoter in the working curve of tRNA Modulator. |
Moreover, strong promoter (T7) of target gene can improve the titration curve of tRNA Modulator, indicating that tRNA Modulator works better under strong target protein promoters. ]] | Moreover, strong promoter (T7) of target gene can improve the titration curve of tRNA Modulator, indicating that tRNA Modulator works better under strong target protein promoters. ]] | ||
| Line 47: | Line 47: | ||
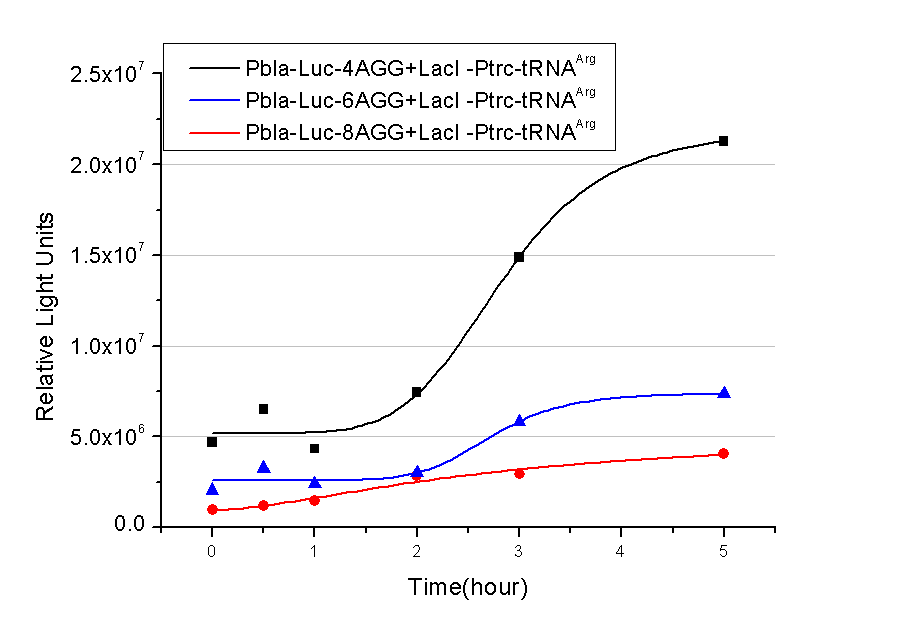
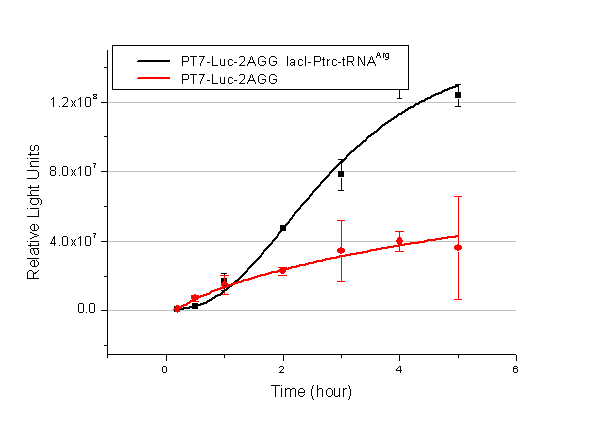
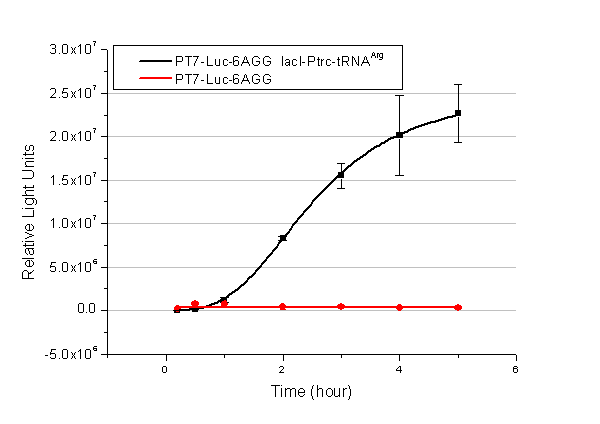
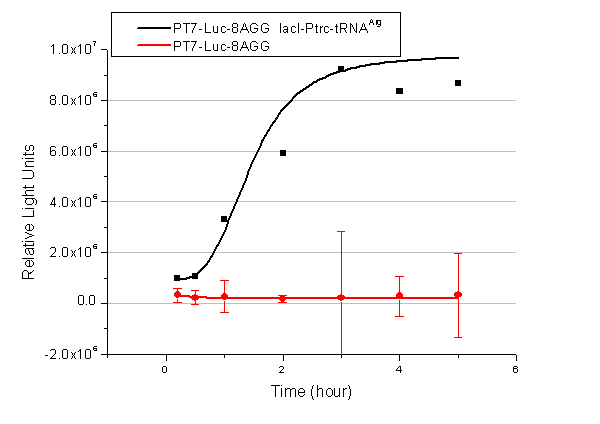
We have tested luciferin reaction in cells. We examined the changes in luciferase enzyme activity over time after rare tRNA expression is induced. The amount of luciferase is reflected indirectly by the bioluminescence emitted from the luciferin reaction. Results are shown below: | We have tested luciferin reaction in cells. We examined the changes in luciferase enzyme activity over time after rare tRNA expression is induced. The amount of luciferase is reflected indirectly by the bioluminescence emitted from the luciferin reaction. Results are shown below: | ||
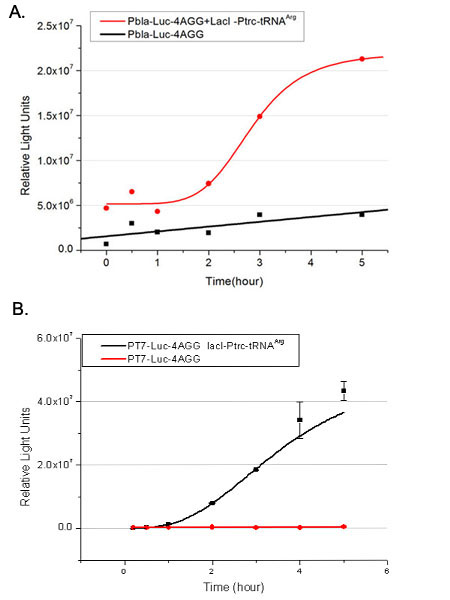
| - | [[image:11-SJTU-compare-4.jpg|frame|center|''Fig. | + | [[image:11-SJTU-compare-4.jpg|frame|center|''Fig.4'' (A)Enzyme activity of luciferase shown by bioluminescence emitted from the luciferin reaction reflecting the working curve of tRNA Modulator ''lacI''-Ptrc-tRNA<sup>Arg</sup> ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567001 BBa_K567001]) under Reporter P''bla''-Luc-4AGG([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567006 BBa_K567006]). (B)Enzyme activity of luciferase shown by bioluminescence emitted from the luciferin reaction reflecting the working curve of tRNA Modulator ''lacI''-Ptrc-tRNA<sup>Arg</sup> ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567001 BBa_K567001]) under Reporter PT7-Luc-4AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567009 BBa_K567009]). The working curve of tRNA Modulator ''lacI''-Ptrc-tRNA<sup>Arg</sup> fits typical titration curve.]] |
Here we use the above two curves as examples to characterize the working curve of our device. Both curves fits typical titration curve, indicating that our device can function as a regulating tool. | Here we use the above two curves as examples to characterize the working curve of our device. Both curves fits typical titration curve, indicating that our device can function as a regulating tool. | ||
Revision as of 17:19, 28 October 2011
 "
"