Team:SJTU-BioX-Shanghai/Project/Application
From 2011.igem.org
(→3. Easy Manipulation) |
(→3. Easy Manipulation) |
||
| Line 43: | Line 43: | ||
*In biosynthetic pathways, feedback can affect protein production greatly. Our device can act as a feedback regulating tool to regulate the biosynthesis of target protein.Target protein and rare tRNA are under the control of the same promoter. Rare tRNA amount can regulate target protein biosynthesis through the rare codons inserted into the gene. | *In biosynthetic pathways, feedback can affect protein production greatly. Our device can act as a feedback regulating tool to regulate the biosynthesis of target protein.Target protein and rare tRNA are under the control of the same promoter. Rare tRNA amount can regulate target protein biosynthesis through the rare codons inserted into the gene. | ||
| - | [[image:11SJTU_App_02.jpg|thumb| | + | [[image:11SJTU_App_02.jpg|thumb|400px|center|application model|''Fig.2'' A Feedback Regulating Tool ]] |
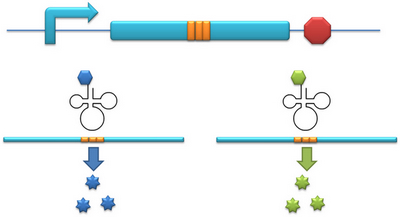
Another factor that influences protein biosynthesis may be the relative amount of two proteins. Our device can serve as a linker to regulate the relative amount of Protein A and Protein B. Rare codons are put into Gene A and Gene B. When rare tRNA is produced, it can influence the amount of both Protein A and Protein B, along with rare codon types and numbers. | Another factor that influences protein biosynthesis may be the relative amount of two proteins. Our device can serve as a linker to regulate the relative amount of Protein A and Protein B. Rare codons are put into Gene A and Gene B. When rare tRNA is produced, it can influence the amount of both Protein A and Protein B, along with rare codon types and numbers. | ||
Revision as of 17:11, 28 October 2011
|
|
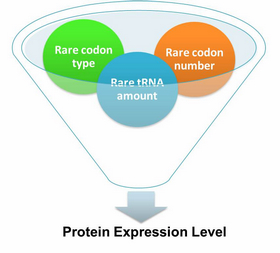
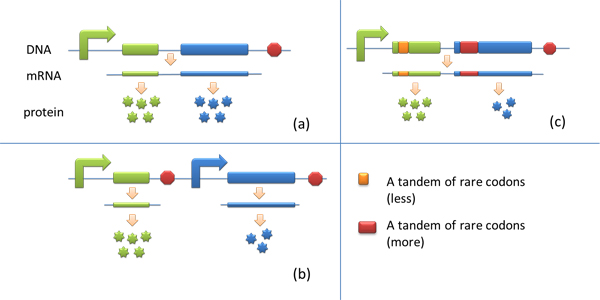
ApplicationI. Expansion of Regulating Tools for Synthetic Biology1. Multi-Level RegulationOur device has expanded the regulating tools for synthetic biology. We control protein biosynthesis through rare codon types, rare codon numbers and rare tRNA amount. Different combinations of these three elements can bring different outcomes in protein expression levels. rare codon types: AGA, AGG, CGA, CUA… rare codon numbers: 1, 2,3, 4,5, 6,7, 8,9, 10… rare tRNA amount: controlled by different strengths of promoters or riboswitch. 2. Direct and Precise RegulationAs a translational regulatory tool, our device achieves more precise tuning of protein expression when compared with transcriptional tools. The controlling elements we use in this device are codons and tRNAs, the major participants of translation process. Thus manipulating these elements exert direct effect on protein biosynthesis. 3. Easy ManipulationOur device is easy to manipulate. Here we offer two potential models for how this device can be used in pathway construction.
Yet to achieve maximum production, each protein amount should be individually regulated. Traditionally we use different promoters to control different genes. (b) However with our device, genes can be put under one single promoter yet producing different amount of protein. (c)
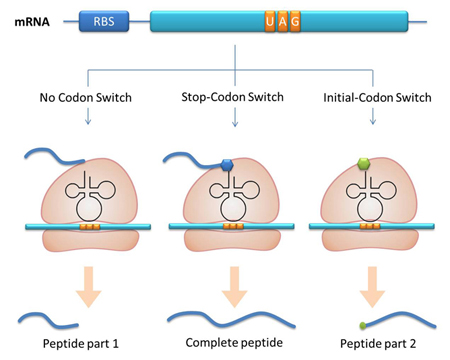
Another factor that influences protein biosynthesis may be the relative amount of two proteins. Our device can serve as a linker to regulate the relative amount of Protein A and Protein B. Rare codons are put into Gene A and Gene B. When rare tRNA is produced, it can influence the amount of both Protein A and Protein B, along with rare codon types and numbers. II. A New Method to Incorporate Point Mutation into ProteinaaRS Modulator can be used as a tool to incorporate point mutation into protein. In our project for instance, we have modified tRNAAsp’s anticodon to base pair with AGG, which is originally the codon for Arg. Then we have modified the AspRS. This modified AspRS can charge Asp to tRNAAsp-AGG. For the protein, codon AGG is originally decoded as Arg yet now it is translated into Asp, thus incorporating point mutation. This method is fit for any other codon and any other tRNAs. III. A New Method to Study the Important Domains of a ProteinInitial-Codon Switch can be used as a tool to study the important domains of a protein. With Initial-Codon Switch, we can start translation at any given site, selectively expressing part of an ORF by introducing a new start codon, a new tRNAfmet, a new aminoacyl-tRNA synthetase and a new 16S rRNA. We replace the first codon of the selected part of the peptide with a rare codon. Here, the rare codon is used as the start codon (Reporter). Concurrently, an altered tRNAmet with anticodon base pairing the rare codon and its corresponding aminoacyl-tRNA synthetase are introduced into the cell (Initial-Codon Switch). In order to guarantee the recognition of ribosome to mRNA, the base pairing between RBS of mRNA and MBS of 16SrRNA should be assured. The 5 nucleotides which are 7 nucleotides upstream of start (rare) codon is selected as the RBS sequence. The gene of an MBS base-pairing the selected RBS sequence is introduced into the 16s rRNA gene, replacing the original MBS. Thus the cell has a new type of ribosome which can recognize the selected RBS. This procedure mimics the original base pairing between RBS and MBS by changing the conserved RBS according to the sequence of the gene. With the design above, we can achieve the selective expression of part of an ORF. |
 "
"