|
|
| (105 intermediate revisions not shown) |
| Line 3: |
Line 3: |
| | <a name='indice'></a> | | <a name='indice'></a> |
| | <h2 class="art-postheader"> | | <h2 class="art-postheader"> |
| - | Parts | + | Characterized Parts |
| | </h2> | | </h2> |
| | <div class="cleared"></div> | | <div class="cleared"></div> |
| Line 10: |
Line 10: |
| | <div class="cleared"></div> | | <div class="cleared"></div> |
| | <div class="art-postcontent"> | | <div class="art-postcontent"> |
| - | | + | <br> |
| | <table id="toc" class="toc"><tr><td><div id="toctitle"><h2>Contents</h2></div> | | <table id="toc" class="toc"><tr><td><div id="toctitle"><h2>Contents</h2></div> |
| | <ul> | | <ul> |
| Line 20: |
Line 20: |
| | <li class="toclevel-2"><a href="#note"><span class="tocnumber">2.1</span> <span class="toctext">Notes on promoter characterization</span></a></li> | | <li class="toclevel-2"><a href="#note"><span class="tocnumber">2.1</span> <span class="toctext">Notes on promoter characterization</span></a></li> |
| | <li class="toclevel-2"><a href="#rbs"><span class="tocnumber">2.2</span> <span class="toctext">RBSs from the community collection</span></a></li> | | <li class="toclevel-2"><a href="#rbs"><span class="tocnumber">2.2</span> <span class="toctext">RBSs from the community collection</span></a></li> |
| - | <li class="toclevel-2"><a href="#pTet"><span class="tocnumber">2.3</span> <span class="toctext">pTet - BBa_R0040</span></a></li> | + | <li class="toclevel-2"><a href="#pLux"><span class="tocnumber">2.3</span> <span class="toctext">p<sub>Lux</sub> - a 3OC<sub>6</sub>-HSL-in PoPs-out device</span></a></li> |
| - | <li class="toclevel-2"><a href="#pLux"><span class="tocnumber">2.4</span> <span class="toctext">pLux - a 3OC6-HSL-in PoPs-out device</span></a></li> | + | <li class="toclevel-2"><a href="#pTet"><span class="tocnumber">2.4</span> <span class="toctext">p<sub>Tet</sub> - BBa_R0040</span></a></li> |
| | <li class="toclevel-2"><a href="#LuxI"><span class="tocnumber">2.5</span> <span class="toctext">LuxI - BBa_C0061</span></a></li> | | <li class="toclevel-2"><a href="#LuxI"><span class="tocnumber">2.5</span> <span class="toctext">LuxI - BBa_C0061</span></a></li> |
| | <li class="toclevel-2"><a href="#AiiA"><span class="tocnumber">2.6</span> <span class="toctext">AiiA - BBa_C0060</span></a></li> | | <li class="toclevel-2"><a href="#AiiA"><span class="tocnumber">2.6</span> <span class="toctext">AiiA - BBa_C0060</span></a></li> |
| Line 27: |
Line 27: |
| | <li class="toclevel-1"><a href="#Improvement"><span class="tocnumber">3</span> <span class="toctext">Sequence debugging</span></a></li> | | <li class="toclevel-1"><a href="#Improvement"><span class="tocnumber">3</span> <span class="toctext">Sequence debugging</span></a></li> |
| | </ul></td></tr></table> | | </ul></td></tr></table> |
| - | </div> | + | </div><br> |
| | + | |
| | + | |
| | + | |
| | + | <br><br> |
| | + | |
| | + | <em> |
| | + | NB: unless differently specified, all tests were performed in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Protocols#MG1655Z1'><em>E. coli</em> MGZ1</a> in M9 supplemented medium at 37°C in low copy plasmid <A HREF="http://partsregistry.org/wiki/index.php/Part: pSB4C5">pSB4C5</a>. |
| | + | </em> |
| | + | <br> |
| | + | <br> |
| | + | |
| | | | |
| | <div class="listcircle"> | | <div class="listcircle"> |
| Line 45: |
Line 56: |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| | <p align='justify'> | | <p align='justify'> |
| - | BBa_J23101 is the reference standard promoter for the computation of RPUs. As discussed in '<a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements'>data analysis</a>' section, RPUs are relative units for the evaluation of promoter strength, based on a mathematical model of the transcription and the translation of a reporter gene. <br> | + | <div align="justify"><p>BBa_J23101 is the reference standard promoter for the computation of RPUs. As discussed in '<a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements'>data analysis</a>' section, RPUs are relative units for the evaluation of promoter strength, based on a mathematical model of the transcription and the translation of a reporter gene.</p> |
| - | The RPUs are supposed to be indepedent on the experimental setup, provided that the reference standard BBa_J23101 must be assayed in the same experimental condition of the studied promoter. <br>It means that if the studied promoter is in a low copy number plasmid and drives the expression of a reporter protein P, J23101 must be assembled in the same vector upstream of the same reporter P.<br> | + | <p>The RPUs are supposed to be indepedent on the experimental setup, provided that the reference standard BBa_J23101 must be assayed in the same experimental condition of the studied promoter. It means that if the studied promoter is in a low copy number plasmid and drives the expression of a reporter protein P, J23101 must be assembled in the same vector upstream of the same reporter P. This approach is in accordance with the philosophy of synthetic biology, based on the concept of 'modularity' of the components. According to this approach, the assembly of basic well characterized modules to build complex circuits allows the prediction of the circuit behavior starting from the knowledge on the basic parts.</p> |
| - | This approach is in accordance with the philosophy of synthetic biology, based on the concept of 'modularity' of the components. According to this approach, the assembly of basic well characterized modules to build complex circuits allows the prediction of the circuit behavior starting from the knowledge on the basic parts. <br> | + | <p>Salis et al. [Nat Biotec, 2009] stated that <em> 'Identical ribosome binding site sequences in different genetic contexts can result in different protein expression levels'</em> and again <em>'It is likely that this absence of modularity is caused by the formation of strong secondary structures between the RBS-containing RNA sequence and one protein coding sequence but not another.'</em></p> |
| - | Salis et al. [Nat Biotec, 2009] stated that | + | |
| - | <em> 'Identical ribosome binding site sequences in different genetic contexts can result in different protein expression levels'</em><br><br> | + | |
| - | and again | + | |
| - | <em>'It is likely that this absence of modularity is caused by the formation of strong secondary structures between the RBS-containing RNA sequence and one protein coding sequence but not another.'</em> <br><br> | + | |
| - | | + | |
| - | | + | |
| - | For this reason, RPUs might not be reliable when comparing the same promoter with different RBSs because of the un-modularity of the RBS.<br>
| + | |
| | | | |
| - | In order to asses what's the effect of RBS 'un-modularity' on RPUs reliability, we have built a set of four constitutive promoters (BBa_J23101) followed by one of the four RBSs tested. These parts were used to evaluate RBS efficiency. Data were collected and analyzed as described in 'Measurements' and 'Data analysis' sections. RPUs and Synthesis rate per cell [AUr] were computed and results are summarized in the table below. | + | <p>For this reason, RPUs might not be reliable when comparing the same promoter with different RBSs because of the un-modularity of the RBS. In order to asses what's the effect of RBS 'un-modularity' on RPUs reliability, we have built a set of four constitutive promoters (BBa_J23101) followed by one of the four RBSs tested. These parts were used to evaluate RBS efficiency. Data were collected and analyzed as described in 'Measurements' and 'Data analysis' sections. RPUs and Synthesis rate per cell [AUr] were computed and results are summarized in the table below.</p> |
| | | | |
| - | <br><br><em> | + | <p><br><em><b>NB</b>: in the RPU computation, the J23101-RBS34-mRFP-TT (<A HREF="http://partsregistry.org/wiki/index.php/Part:BBa_J23101">BBa_J23101</a>) in low copy plasmid <A HREF="http://partsregistry.org/wiki/index.php/Part:pSB4C5">pSB4C5</a> construct has been considered as the reference standard. With this assumption, RPUs are identical to the estimated RBS efficiency.</em></p></div> |
| - | <b>NB</b>: in the RPU computation, the J23101-RBS34-mRFP-TT construct has been considered as the reference standard. With this assumption, RPUs are identical to the estimated RBS efficiency. </em> | + | |
| | | | |
| | <table align='center'><tr><td width='50%' > | | <table align='center'><tr><td width='50%' > |
| Line 74: |
Line 77: |
| | </td> | | </td> |
| | <td class='row'>122 [13.23]</td> | | <td class='row'>122 [13.23]</td> |
| - | <td class='row'>2.56 [0.27]</td> | + | <td class='row'>2.45 [0.27]</td> |
| | </tr> | | </tr> |
| | <tr><td class='row'>RBS31 | | <tr><td class='row'>RBS31 |
| Line 92: |
Line 95: |
| | </tr> | | </tr> |
| | </table></td> | | </table></td> |
| - | Data are provided as average [Standard error].<br><br> | + | <div align="center">Data are provided as average [Standard error].</div><br> |
| | <td width='50%' > | | <td width='50%' > |
| - | <div style="text-align:justify"><div class="thumbinner" width='80%'><a href="File:UNIPV_RPU_J101.png" class="image"><img alt="RPUs of J23101 promoter with different RBSs" src="https://static.igem.org/mediawiki/2011/b/bb/UNIPV_RPU_J101.png" class="thumbimage" width="80%"></a></div></div> | + | <div style="text-align:justify"><div class="thumbinner" width='80%'><a href="https://static.igem.org/mediawiki/2011/b/bb/UNIPV_RPU_J101.png" class="image"><img alt="RPUs of J23101 promoter with different RBSs" src="https://static.igem.org/mediawiki/2011/b/bb/UNIPV_RPU_J101.png" class="thumbimage" width="80%"></a></div></div> |
| | </td> | | </td> |
| | </tr></table> | | </tr></table> |
| | <br><br> | | <br><br> |
| - | On the other hand, the hypothesis of RBS modularity depending on the promoter is accepted, the J23101-RBSx series we have provided can be used as a library of ready-to-use reference standard for RPU evaluation, that allows to depurate RPU measurement from RBS effect, thus providing only the promoter strength. | + | <div align="justify">On the other hand, if the hypothesis of RBS modularity depending on the promoter is accepted, the J23101-RBSx series we have provided can be used as a library of ready-to-use reference standard for RPU evaluation, that allows to depurate RPU measurement from RBS effect, thus providing only the promoter strength.</div> |
| | | | |
| | </p> | | </p> |
| Line 115: |
Line 118: |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516212 "> BBa_K516212 </a> (wiki name: E15 ) pTet-RBS32-LuxI </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516212 "> BBa_K516212 </a> (wiki name: E15 ) pTet-RBS32-LuxI </li> |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516214 "> BBa_K516214 </a> (wiki name: E16 ) pTet-RBS34-LuxI </li></ul></ol> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516214 "> BBa_K516214 </a> (wiki name: E16 ) pTet-RBS34-LuxI </li></ul></ol> |
| | + | |
| | + | <br><br> |
| | + | <p align='justify'><em>Though these parts don't have a transcriptional terminator, they have been characterized in low copy plasmid <A HREF="http://partsregistry.org/wiki/index.php/Part:pSB4C5">pSB4C5</a>, that contains the BBa_B0054 terminator. This choice is motivated by the need to reproduce the exact experimental context of the final circuit, as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Solution#Circuit_design'>solution section</a>. |
| | + | </em></p> |
| | + | <br><br> |
| | + | <p align='justify'> |
| | + | LuxI has been characterized in terms of enzymatic activity under the regulation of p<sub>Tet</sub> promoter. |
| | + | </p> |
| | + | <p align='justify'> |
| | + | K<sub>M,LuxI</sub> and V<sub>max</sub> parameters representing its activity have been estimated and the promoter strength (represented by a synthetic parameter α<sub>pTet</sub> for every pTet-RBS combination) at full induction (100 ng/ml) has been estimated too with a simultaneous fitting of the available data. |
| | + | |
| | + | </p> |
| | + | |
| | + | |
| | + | <p align='justify'> |
| | + | |
| | + | <p align='justify'> |
| | + | |
| | + | LuxI has been characterized through the Biosensor BBa_T9002 (see <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#introduction_to_T9002'>modelling section</a>). |
| | + | <br>The HSL synthesis rate has been evaluated according to the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>model equations</a>, properly adjusted. In particular, the ODE system describing the behavior of this measurement circuit is reported here: </p> |
| | + | |
| | + | <div style='text-align:justify'><div class="thumbinner" style="width: 850px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2011/a/a6/Pc_luxi.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/a/a6/Pc_luxi.jpg" class="thumbimage" height="70%" width="68%"></a></div></div> |
| | + | |
| | + | |
| | + | |
| | + | <p align='justify'> |
| | + | Since the measurement systems are only assayed in the exponential growth phase, in the equation (3) N < N<sub>max</sub> is assumed. |
| | + | The parameters N<sub>max</sub>, μ and γ<sub>HSL</sub> (see the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Results'>Results section</a> for more details) are known and summarized in the table below:</p> |
| | + | |
| | + | <div align="center"> |
| | + | <table align="center" class='data' title='parameter value'> |
| | + | <tr> |
| | + | <td class="row"><b>N<sub>max</sub></sub></b></td> |
| | + | <td class="row"><b>μ</b></td> |
| | + | <td class="row"><b>γ<sub>HSL</sub></b></td> |
| | + | </tr> |
| | + | <tr> |
| | + | <td class='row' >1*10<sup>9</sup></td> |
| | + | <td class='row' >0.004925</td> |
| | + | <td class='row' >0</td> |
| | + | </table> |
| | + | </p> |
| | + | </div> |
| | + | |
| | + | <p align='justify'> |
| | + | The parameters V<sub>max</sub>, k<sub>M,LuxI</sub> and α<sub>RBSx</sub> were estimated with a simultaneous fitting of the data collected as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#LuxI'>measurement section</a> for the four measurement parts <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetLuxI'>pTet-RBSx-LuxI-TT</a> assayed by <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#T9002'>BBa_T9002 biosensor</a> section. |
| | + | |
| | + | |
| | + | <div align="justify"><div class="thumbinner" style="width: 850px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2011/4/40/Luxi_prod.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/4/40/Luxi_prod.jpg" class="thumbimage" width="85%" height="50%"></a></div></div> |
| | + | |
| | + | |
| | + | <div align="justify"><p>The estimated parameters for the enzymatic activity of LuxI are reported in the table below:</p></div> |
| | + | |
| | + | |
| | + | <div align="center"> |
| | + | <table align="center" class='data'> |
| | + | <tr> |
| | + | <td class='row'><b>V<sub>max</sub></b></td> |
| | + | <td class='row'><b>k<sub>M,LuxI</sub></b></td> |
| | + | <td class='row'><b>α<sub>B0030</sub></b></td> |
| | + | <td class='row'><b>α<sub>B0031</sub></b></td> |
| | + | <td class='row'><b>α<sub>B0032</sub></b></td> |
| | + | <td class='row'><b>α<sub>B0034</sub></b></td> |
| | + | </tr> |
| | + | <tr><td class='row'>3.56*10<sup>-9</sup></td> |
| | + | <td class='row'>6.87*10<sup>3</sup></td> |
| | + | <td class='row'>87</td> |
| | + | <td class='row'>8.5</td> |
| | + | <td class='row'>ND</td> |
| | + | <td class='row'>252</td> |
| | + | </tr></table> |
| | + | </div> |
| | + | <br> |
| | + | </p> |
| | + | </p> |
| | + | |
| | + | |
| | + | </p> |
| | + | |
| | + | |
| | + | <p align='justify'> |
| | + | The collected data have been used to identify the parameters of our model. Despite the data-poor context, the model predictions fit the experimental data, thus demonstrating that the equation that models the HSL synthesis by LuxI is a good approximation of real processes. |
| | + | </p> |
| | | | |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| Line 121: |
Line 211: |
| | | | |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| - | <!-- pLuxAiiA description --> | + | <!-- pTetAiiA description --> |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| | | | |
| - | <a name='pTetAiiA'></a><h2>AiiA expression cassette driven by aTc-inducible pTet promoter</h2> | + | <a name='pTetAiiA'></a><h2>AiiA expression cassette driven by aTc-inducible p<sub>Tet</sub> promoter</h2> |
| | <ol> | | <ol> |
| | <ul> | | <ul> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516220 "> BBa_K516220 </a> (wiki name: E24 ) pTet-RBS30-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516220 "> BBa_K516220 </a> (wiki name: E24 ) p<sub>Tet</sub>-RBS30-AiiA-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516221 "> BBa_K516221 </a> (wiki name: E25 ) pTet-RBS31-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516221 "> BBa_K516221 </a> (wiki name: E25 ) p<sub>Tet</sub>-RBS31-AiiA-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516222 "> BBa_K516222 </a> (wiki name: E26 ) pTet-RBS32-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516222 "> BBa_K516222 </a> (wiki name: E26 ) p<sub>Tet</sub>-RBS32-AiiA-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516224 "> BBa_K516224 </a> (wiki name: E27 ) pTet-RBS34-AiiA-TT </li></ul></ol> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516224 "> BBa_K516224 </a> (wiki name: E27 ) p<sub>Tet</sub>-RBS34-AiiA-TT </li></ul></ol> |
| | | | |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| | | | |
| | + | <p aling="justify"> |
| | + | The AiiA enzyme activity has been characterized under the regulation of p<sub>tet</sub> promoter, assaying its enzymatic activity. |
| | + | Similar to LuxI, a system of differential equations (referring to <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>model equations</a>) has been derived. |
| | + | </p> |
| | | | |
| | + | <div style='text-align:justify'> |
| | + | <div class="thumbinner" style="width: 850px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg" class="thumbimage" height="70%" width="68%"></a> |
| | + | </div></div> |
| | | | |
| | + | <p align='justify'> |
| | + | The parameters k<sub>cat</sub>, k<sub>M,AiiA</sub> and α<sub>RBSx</sub> would have been estimated with a simultaneous fitting of the data collected as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#AiiA'>measurement section</a> for the four measurement parts <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetAiiA'>p<sub>Tet</sub>-RBSx-AiiA-TT</a> assayed by <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#T9002'>BBa_T9002 biosensor</a> section. |
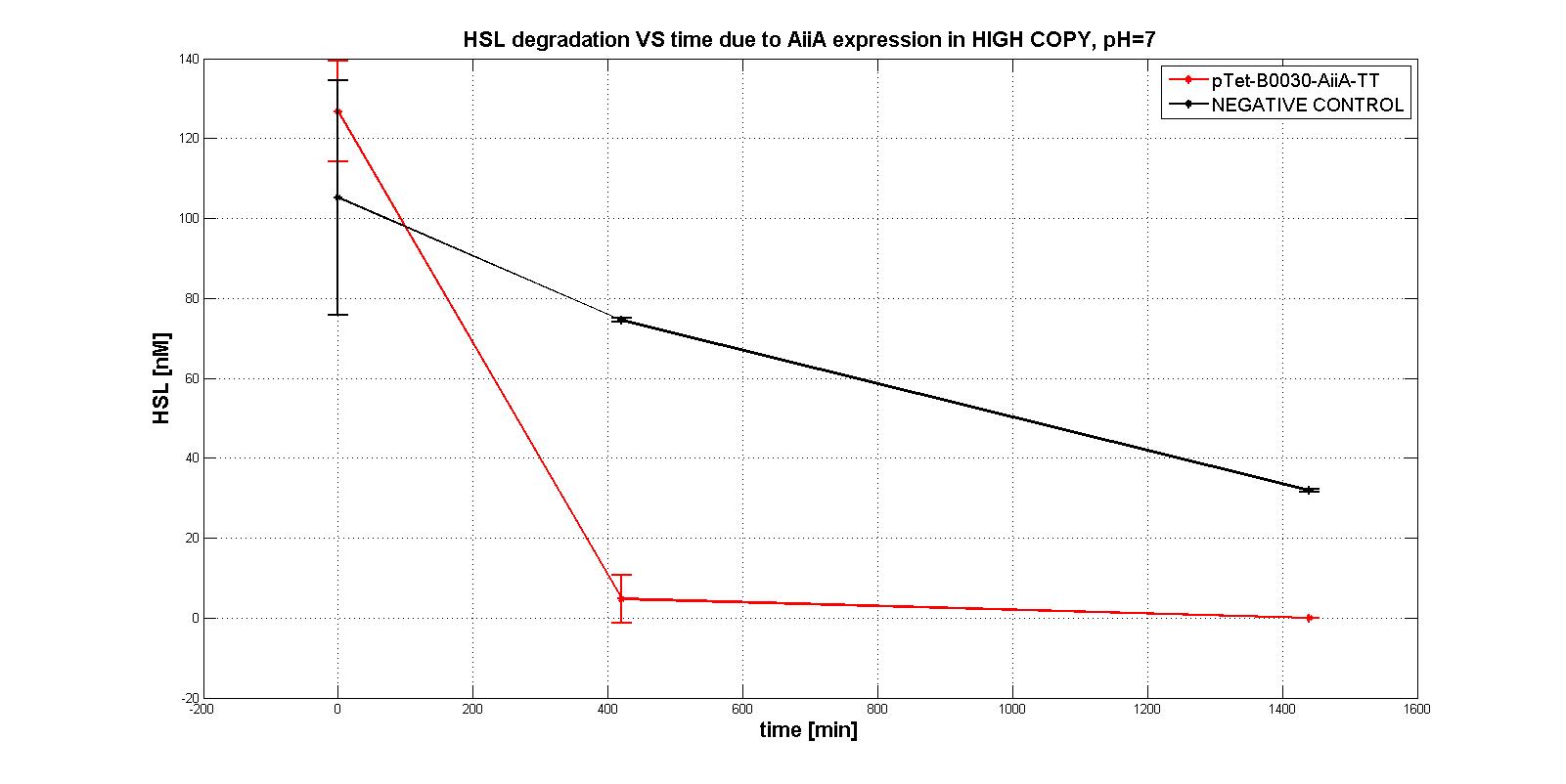
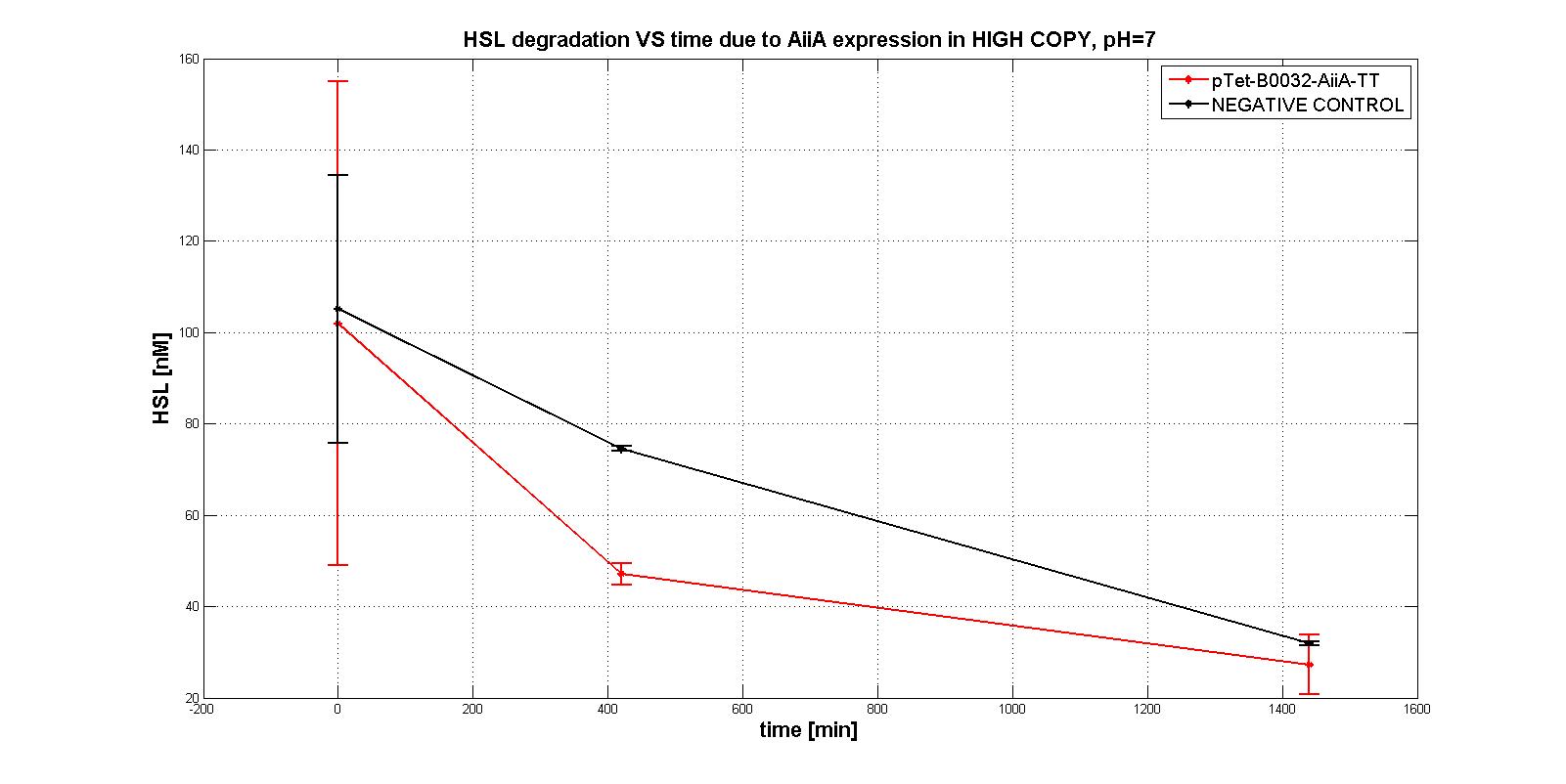
| | + | Unfortunately, their estimation revealed difficult.<br> |
| | + | In the first experiments with the measurement system <a href="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg">p<sub>Tet</sub>-RBSx-AiiA-TT</a> in LOW-COPY at pH=7 no degradation of HSL was observed. The collected data are shown in the figure below. HSL degradation is identical in the measurement system and in the negative control after 21 hours. |
| | + | |
| | + | |
| | + | <table width='100%'><tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/6/69/Aiia_LC_B0030.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/6/69/Aiia_LC_B0030.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/6/63/Aiia_LC_B0031.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/6/63/Aiia_LC_B0031.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | <tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/f/f5/Aiia_LC_B0032.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/f/f5/Aiia_LC_B0032.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/8/8d/Aiia_LC_B0034.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/8/8d/Aiia_LC_B0034.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | </table> |
| | + | |
| | + | |
| | + | <div align="justify">Further investigation on the enzyme were performed in more suitable conditions, to evaluate its intrinsic activity. AiiA activity was investigated in HIGH COPY plasmid in <em>E. coli</em> TOP10, in order to understand if the enzyme worked. In this case, a significant difference in degradation between p<sub>Tet</sub>-RBSx-AiiA-TT and the negative control was observed, also just after 7 hours. That was the proof of the good functioning (in HIGH COPY) of the enzyme.</div> |
| | + | |
| | + | |
| | + | <table width='100%'><tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/6/6c/Aiia_HC_B0030.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/6/6c/Aiia_HC_B0030.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/e/ec/Aiia_HC_B0031.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/e/ec/Aiia_HC_B0031.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | <tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/b/b2/Aiia_HC_B0032.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/b/b2/Aiia_HC_B0032.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/d/d7/Aiia_HC_B0034.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/d/d7/Aiia_HC_B0034.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | </table> |
| | + | |
| | + | |
| | + | <div align="justify"> |
| | + | Experiments on these parts gave us the opportunity to characterize only the activity of the enzyme in <em>E. COLI</em> TOP10 in high copy number plasmid, providing only some information about the order of magnitude of the model parameters, which has been designed to work in <em>E. COLI</em> MGZ1 in low copy number plasmid.</div> |
| | + | |
| | + | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| | | | |
| | <a name='Existing'></a><h1>Existing parts</h1> | | <a name='Existing'></a><h1>Existing parts</h1> |
| Line 141: |
Line 299: |
| | <a name='note'></a><h2>Notes for promoter characterization</h2> | | <a name='note'></a><h2>Notes for promoter characterization</h2> |
| | <p align='justify'> | | <p align='justify'> |
| - | Inducible and constitutive promoters were assembled upstream of different coding sequences containing an RBS from the Community collection. | + | <p>Inducible and constitutive promoters were assembled upstream of different coding sequences containing an RBS from the Community collection.</p> |
| - | <br><br> | + | <p>The assembled RBSs are:</p> |
| - | The assembled RBSs are: | + | <br> |
| - | <br><br> | + | |
| | <div align='center'> | | <div align='center'> |
| | <table class='data'> | | <table class='data'> |
| Line 154: |
Line 311: |
| | </table></div> | | </table></div> |
| | <br> | | <br> |
| - | For an inducible device, the RBS variation has the purpose to stretch the induction curve, thus modulating its PoPs-OUT range. | + | <div align="justify"><p>For an inducible device, the RBS variation has the purpose to stretch the induction curve, thus modulating its PoPs-OUT range.</p> |
| - | <br><br> | + | <p>The complex RBS-promoter acts as a whole regulatory element and determines the amount of translated protein. |
| - | The complex RBS-promoter acts as a whole regulatory element and determines the amount of translated protein. | + | RBSs have been reported to have an un-modular behavior, since the translational efficiency is not independent on the coding sequences, but variates as an effect of different mRNA structure stability [Salis et al., Nat Biotec, 2009]. It is not possible to separate the effects of the sole promoter and of the sole RBS on the total amount/activity of gene product (in this case study, mRFP).</p> |
| - | RBSs have been reported to have an un-modular behavior, since the translational efficiency is not independent on the coding sequences, but variates as an effect of different mRNA structure stability [Salis et al., Nat Biotec, 2009]. <br> | + | <p>For this reason, every combination 'Promoter+RBS' was studied as a different regulatory element. Regulatory elements were characterized using mRFP reporter protein for different RBSs in terms of Synthesis rate per Cell (<b>S<sub>cell</sub></b>) and <b>R.P.U.s</b> (Relative Promoter Units) as explained in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements'>measurements</a> section.</p> |
| - | It is not possible to separate the effects of the sole promoter and of the sole RBS on the total amount/activity of gene product (in this case study, mRFP). <br><br> | + | |
| - | For this reason, every combination 'Promoter+RBS' was studied as a different regulatory element. | + | |
| - | Regulatory elements were characterized using mRFP reporter protein for different RBSs in terms of Synthesis rate per Cell (<b>S<sub>cell</sub></b>) and <b>R.P.U.s</b> (Relative Promoter Units) as explained in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements'>measurements</a> section. | + | |
| - | | + | |
| | </p> | | </p> |
| - | | + | <p>Operative parameters of the promoter are derived from the estimated Hill equations obtained by <em>nonlinear least squares</em> fitting (<em>lsqnonlin</em> Matlab routine) of the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>Hill function</a> expressed in RPUs:</p><p></p> |
| - | <p> | + | <p><ol><ul><li><b> |
| - | Operative parameters of the promoter are derived from the estimated Hill equations obtained by <em>nonlinear least sqares</em> fitting (<em>lsqnonlin</em> Matlab routine) of the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>Hill function</a> expressed in RPUs : | + | RPU<sub>max</sub></b> is equal to the α and represents the maximum promoter activity</p> |
| - | <br> | + | </li><p><li><b> |
| - | <ol><ul><li><b> | + | RPU<sub>min</sub></b> is equal to the α * δ represents the minimum promoter activity</p> |
| - | RPU<sub>max</sub></b> is equal to the α and represents the maximum promoter activity, | + | </li><p><li> |
| - | </li><li><b> | + | <b>Switch point</b> is computed as the abscissa of the inflection point of the Hill curve and it is representative of the position of linear region</p> |
| - | RPU<sub>min</sub></b> is equal to the α * δ represents the minimum promoter activity, | + | |
| - | </li><li> | + | |
| - | <b>Switch point</b> is computed as the abscissa of the inflection point of the Hill curve and it is representative of the position of linear region, | + | |
| | </li> | | </li> |
| - | <li> | + | <p><li> |
| - | <b>Linearity boundaries</b> are determined as the intersection between the tangent line to the inflection point and the upper and lower horizontal boundaries of the Hill curve.</li> | + | <b>Linearity boundaries</b> are determined as the intersection between the tangent line to the inflection point and the upper and lower horizontal boundaries of the Hill curve.</div></li></p> |
| | </ul></ol> | | </ul></ol> |
| | </p> | | </p> |
| Line 186: |
Line 336: |
| | <a name='rbs'></a><h2>RBSs</h2> | | <a name='rbs'></a><h2>RBSs</h2> |
| | <p align='justify'> | | <p align='justify'> |
| - | The complex RBS-promoter acts as a whole regulatory element and determines the amount of translated protein. RBSs have been reported to have an un-modular behavior, since the translational efficiency is not independent on the coding sequences, but variates as an effect of different mRNA structure stability [Salis et al., Nat Biotec, 2009]. In addition, it is not possible to separate the effects of the sole promoter and of the sole RBS on the total amount of mRFP produced. | + | <p><div align="justify">The complex RBS-promoter acts as a whole regulatory element and determines the amount of translated protein. RBSs have been reported to have an un-modular behavior, since the translational efficiency is not independent on the coding sequences, but variates as an effect of different mRNA structure stability [Salis et al., Nat Biotec, 2009]. In addition, it is not possible to separate the effects of the sole promoter and of the sole RBS on the total amount of protein produced. |
| - | For this reason, every combination 'Promoter+RBS' was studied as a different regulatory element. | + | For this reason, every combination 'Promoter+RBS' was studied as a different regulatory element.</p> |
| - | The evaluation of RBS efficiency can be performed in a very intuitive fashion:<br> | + | <p>The evaluation of RBS efficiency can be performed in a very intuitive fashion:</p> |
| - | <ul> | + | <ol> |
| - | <li>1. select the RBSs you want to study, </li> | + | <li>select the RBSs you want to study</li> |
| - | <li>2. assemble them in a Promoter - XX - Coding sequence circuit, </li></ul></ol> | + | <li>assemble them in a Promoter - XX - Coding sequence circuit</li> |
| | | | |
| | <table align='center' width='100%'> | | <table align='center' width='100%'> |
| Line 202: |
Line 352: |
| | </table> | | </table> |
| | | | |
| - | <ul>
| + | |
| - | <li>3. measure the output of the circuits and calculate the RBS efficiency as the ratio of the output relative to the output of the circuit with the standard RBS. </li> | + | <li>measure the output of the circuits and calculate the RBS efficiency as the ratio of the output relative to the output of the circuit with the standard RBS (<a href='http://partsregistry.org/wiki/index.php/Part: BBa_B0034'>BBa_B0034</a>). </li></div></ol><br> |
| - | </ul></ol><br> | + | |
| - | This simple measurement system allows the quantification of RBS efficiency depending on the whole measurement system (i.e.: promoter and encoded gene). Today it has not still been completely validated the hypothesis that every functional module in a genetic circuit maintains its behavior when assembled in a complex circuits, even if many researchers implicitly accept this hypothesis when performing characterization experiments. <br> | + | |
| - | To rationally assess the impact that this hypothesis has on the genetic circuit design and fine tuning, several measurement systems were built to evaluate the dependance of RBS modularity from the promoter or the coding sequence separately.<br> | + | <div align="justify"><p>This simple measurement system allows the quantification of RBS efficiency depending on the experimental context (i.e.: promoter and encoded gene). Today it has not still been completely validated the hypothesis that every functional module in a genetic circuit maintains its behavior when assembled in complex circuits, even if many researchers implicitly accept this hypothesis when performing characterization experiments.</p> |
| - | In particular, in order to investigate if RBS efficiency depends on the promoter, the same coding devices (RBSx-RFP-TT) were assembled downstream of different promoters (J23101, pTet, pLux). Measuring the system output and evaluating the RBS efficiency. The results are summarized in the table below:<br><br> | + | <p>To rationally assess the impact that this hypothesis has on the genetic circuit design and fine tuning, several measurement systems were built to evaluate the dependance of RBS modularity from the promoter or the coding sequence separately.</p> |
| - | <table class='data' width='70%'><tr> | + | <p>In particular, in order to investigate if RBS efficiency depends on the promoter, the same coding devices (RBSx-RFP-TT) were assembled downstream of different promoters (J23101, p<sub>Tet</sub>, p<sub>Lux</sub>). The system output was measured and the RBS efficiency evaluated. The results are summarized in the table below:</div></p> |
| | + | <br> |
| | + | <div align="center"><table class='data' width='70%'><tr> |
| | <td class='row'><b>RBS</b></td> | | <td class='row'><b>RBS</b></td> |
| - | <td class='row'><b>eff<sub>pLux</sub></b><sup>*</sup></td> | + | <td class='row'><b>eff<sub>p<sub>Lux</sub></sub></b><sup>*</sup></td> |
| - | <td class='row'><b>eff<sub>pTet</sub></b><sup>*</sup></td> | + | <td class='row'><b>eff<sub>p<sub>Tet</sub></sub></b><sup>*</sup></td> |
| | <td class='row'><b>eff<sub>J23101</sub></b><sup>**</sup></td> | | <td class='row'><b>eff<sub>J23101</sub></b><sup>**</sup></td> |
| | <td class='row'><b>Declared efficiency</b></td> | | <td class='row'><b>Declared efficiency</b></td> |
| Line 222: |
Line 374: |
| | </tr><tr> | | </tr><tr> |
| | <td class='row'>B0034</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td> | | <td class='row'>B0034</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td> |
| - | </tr></table> | + | </tr></table></div> |
| | | | |
| | <br> | | <br> |
| - | On the other end, to investigate the dependance of RBS modularity on the coding sequence, the same regulatory elements (pTet-RBSx) were assembled upstream of different encoded gene (mRFP, AiiA and LuxI). RBS efficiency was assessed and the results are summarized in the table below:<br><br> | + | <div align="justify">On the other hand, to investigate the dependance of RBS modularity on the coding sequence, the same regulatory elements (p<sub>Tet</sub>-RBSx) were assembled upstream of different encoded gene (mRFP, AiiA and LuxI). RBS efficiency was assessed and the results are summarized in the table below:</div><br> |
| | | | |
| - | <table class='data' width='70%'><tr> | + | <div align="center"><table class='data' width='70%'><tr> |
| | <td class='row'><b>RBS</b></td> | | <td class='row'><b>RBS</b></td> |
| | <td class='row'><b>eff<sub>mRFP</sub></b><sup>**</sup></td> | | <td class='row'><b>eff<sub>mRFP</sub></b><sup>**</sup></td> |
| Line 241: |
Line 393: |
| | </tr><tr> | | </tr><tr> |
| | <td class='row'>B0034</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td> | | <td class='row'>B0034</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td><td class='row'>1</td> |
| - | </tr></table> | + | </tr></table></div> |
| | + | <br> |
| | <p align='justify'> | | <p align='justify'> |
| - | <sup>*</sup> The RBS efficiency for inducible devices expressing mRFP was estimated as the ratio of the AUCs (Area under the curve) of the induction curve of the system with the studied RBS and the B0034 reference: AUC<sub>P, RBSx</sub>/AUC<sub>P, B0034</sub><br> | + | <div align="justify"><p><sup>*</sup> The RBS efficiency for inducible devices expressing mRFP was estimated as the ratio of the AUCs (Area under the curve) of the induction curve of the system with the studied RBS and the B0034 reference: AUC<sub>P, RBSx</sub>/AUC<sub>P, B0034</sub></p> |
| - | <sup>**</sup> The RBS efficiency for constitutive promoters expressing mRFP was computed as the ratio between S<sub>cell<sub>P, RBSx</sub></sub>/S<sub>cell<sub>P, B0034</sub></sub><br> | + | <p><sup>**</sup> The RBS efficiency for constitutive promoters expressing mRFP was computed as the ratio between S<sub>cell<sub>P, RBSx</sub></sub>/S<sub>cell<sub>P, B0034</sub></sub></p> |
| - | <sup>***</sup> The RBS efficiency for pTet promoter driving the expression of AiiA enzyme was computed as the ratio α<sub>pTet, RBSx</sub>/α<sub>pTet, B0034</sub> estimated for the measurement system pTet-RBSx-AiiA-TT. α<sub>pTet</sub> was estimated as described <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#AiiA'>here</a>. pTet was tested at full induction (100 ng/ml).<br> | + | <p><sup>***</sup> The RBS efficiency for p<sub>Tet</sub> promoter driving the expression of AiiA enzyme was computed as the ratio between the percentage of degraded HSL after 4 hours of the studied system and the reference B0034. p<sub>Tet</sub> was tested at full induction (100 ng/ml).</p> |
| - | <sup>****</sup>The RBS efficiency for promoters driving the expression of LuxI was computed as the ratio α<sub>pTet, RBSx</sub>/α<sub>pTet, B0034</sub> estimated from the measurement systems pTet-RBSx-LuxI. α<sub>pTet</sub> was estimated as described <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#LuxI'>here</a>. pTet was tested at full induction (100 ng/ml).<br> | + | <p><sup>****</sup> The RBS efficiency for promoters driving the expression of LuxI was computed as the ratio α<sub>p<sub>Tet</sub>, RBSx</sub>/α<sub>p<sub>Tet</sub>, B0034</sub> estimated from the measurement systems p<sub>Tet</sub>-RBSx-LuxI. α<sub>p<sub>Tet</sub></sub> was estimated as described <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#LuxI'>here</a>. p<sub>Tet</sub> was tested at full induction (100 ng/ml).</p></div> |
| | | | |
| | <br> | | <br> |
| | </p> | | </p> |
| - | <br><br>
| |
| | The parts we used to characterize the RBSs are listed here: | | The parts we used to characterize the RBSs are listed here: |
| | <ol> | | <ol> |
| Line 256: |
Line 408: |
| | <ul> | | <ul> |
| | <li>J23101</li> | | <li>J23101</li> |
| - | <ul> | + | <ul class="disc"> |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516130 "> BBa_K516130 </a> J101-RBS30-RFP-TT </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516130 "> BBa_K516130 </a> J101-RBS30-RFP-TT </li> |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516131 "> BBa_K516131 </a> J101-RBS31-mRFP-TT </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516131 "> BBa_K516131 </a> J101-RBS31-mRFP-TT </li> |
| Line 262: |
Line 414: |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_J23101 "> BBa_J23101 </a> J101-RBS34-mRFP-TT </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_J23101 "> BBa_J23101 </a> J101-RBS34-mRFP-TT </li> |
| | </ul> | | </ul> |
| - | <li>pTet</li> | + | <li>p<sub>Tet</sub></li> |
| - | <ul> | + | <ul class="disc"> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516230 "> BBa_K516230 </a> pTet-RBS30-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516230 "> BBa_K516230 </a> p<sub>Tet</sub>-RBS30-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516231 "> BBa_K516231 </a> pTet-RBS31-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516231 "> BBa_K516231 </a> p<sub>Tet</sub>-RBS31-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> pTet-RBS32-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> p<sub>Tet</sub>-RBS32-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_I13521 "> BBa_I13521 </a> pTet-RBS34-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_I13521 "> BBa_I13521 </a> p<sub>Tet</sub>-RBS34-mRFP-TT </li> |
| | </ul> | | </ul> |
| - | <li>pLux</li> | + | <li>p<sub>Lux</sub></li> |
| - | <ul> | + | <ul class="disc"> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516330 "> BBa_K516330 </a> pLambda-RBS30-LuxR-T-pLux-RBS30-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516330 "> BBa_K516330 </a> pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS30-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516331 "> BBa_K516331 </a> pLambda-RBS30-LuxR-T-pLux-RBS31-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516331 "> BBa_K516331 </a> pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS31-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516332 "> BBa_K516332 </a> pLambda-RBS30-LuxR-T-pLux-RBS32-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516332 "> BBa_K516332 </a> pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS32-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516334 "> BBa_K516334 </a> pLambda-RBS30-LuxR-T-pLux-RBS34-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516334 "> BBa_K516334 </a> pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS34-mRFP-TT </li> |
| | </ul> | | </ul> |
| | </ul> | | </ul> |
| - | <li>pTet driving the expression of different genes</li> | + | <li>p<sub>Tet</sub> driving the expression of different genes</li> |
| | <ul><li>mRFP</li> | | <ul><li>mRFP</li> |
| - | <ul> | + | <ul class="disc"> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516230 "> BBa_K516230 </a> pTet-RBS30-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516230 "> BBa_K516230 </a> p<sub>Tet</sub>-RBS30-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516231 "> BBa_K516231 </a> pTet-RBS31-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516231 "> BBa_K516231 </a> p<sub>Tet</sub>-RBS31-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> pTet-RBS32-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> p<sub>Tet</sub>-RBS32-mRFP-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_I13521 "> BBa_I13521 </a> pTet-RBS34-mRFP-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_I13521 "> BBa_I13521 </a> p<sub>Tet</sub>-RBS34-mRFP-TT </li> |
| | </ul> | | </ul> |
| | <li>AiiA</li> | | <li>AiiA</li> |
| - | <ul> | + | <ul class="disc"> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516220 "> BBa_K516220 </a> pTet-RBS30-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516220 "> BBa_K516220 </a> p<sub>Tet</sub>-RBS30-AiiA-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516221 "> BBa_K516221 </a> pTet-RBS31-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516221 "> BBa_K516221 </a> p<sub>Tet</sub>-RBS31-AiiA-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516222 "> BBa_K516222 </a> pTet-RBS32-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516222 "> BBa_K516222 </a> p<sub>Tet</sub>-RBS32-AiiA-TT </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516224 "> BBa_K516224 </a> pTet-RBS34-AiiA-TT </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516224 "> BBa_K516224 </a> p<sub>Tet</sub>-RBS34-AiiA-TT </li> |
| | </ul> | | </ul> |
| | <li>LuxI</li> | | <li>LuxI</li> |
| - | <ul> | + | <ul class="disc"> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516210 "> BBa_K516210 </a> pTet-RBS30-LuxI </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516210 "> BBa_K516210 </a> p<sub>Tet</sub>-RBS30-LuxI </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516211 "> BBa_K516211 </a> pTet-RBS31-LuxI </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516211 "> BBa_K516211 </a> p<sub>Tet</sub>-RBS31-LuxI </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516212 "> BBa_K516212 </a> pTet-RBS32-LuxI </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516212 "> BBa_K516212 </a> p<sub>Tet</sub>-RBS32-LuxI </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516214 "> BBa_K516214 </a> pTet-RBS34-LuxI </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516214 "> BBa_K516214 </a> p<sub>Tet</sub>-RBS34-LuxI </li> |
| | </ul></ul> | | </ul></ul> |
| | </ul></ol> | | </ul></ol> |
| Line 303: |
Line 455: |
| | | | |
| | </p> | | </p> |
| | + | <p align='justify'> |
| | + | The results reported in the table suggest that the RBS efficiency ranking is not always maintained. In particular, for the different promoters driving the expression of mRFP, the ranking of the declared efficiencies is maintained for p<sub>Lux</sub>, but not for p<sub>Tet</sub> and J23101. The RBS B0030 results to be the most efficient for both J23101 and p<sub>Tet</sub>, but not for p<sub>Lux</sub> (NB: this effect might be due to an effective non-modularity of RBS, but also to saturating phenomena occurring for this very strong promoter at full induction). RBS B0031 always shows a very low efficiency, while B0032 an intermediate efficiency between B0031 and the stronger RBSs B0030 and B0034. <br> |
| | + | For what concerns the encoded gene variation, more significant differences can be observed. RBS B0030 has the higher efficiency only for mRFP, while the values for AiiA and LuxI are similar (~0.5). Unexpectedly, the weak RBS B0031 has a higher efficiency with AiiA gene (0.83), while with mRFP and LuxI. With B0032 no activity was observed for LuxI, while for AiiA and mRFP the results are quite consistent with the one reported above. |
| | + | </p> |
| | + | <p align='justify'> |
| | + | These results are encouraging: though the partial non-modularity of RBS with the encoded gene is confirmed, the hypothesis of modularity with the promoter is to some extent confirmed. Three classes of efficiencies were identified: |
| | + | <ul> |
| | + | <li>low efficiency RBS (B0031)</li> |
| | + | <li>medium efficiency RBS (B0032)</li> |
| | + | <li>high efficiency RBSs (B0030, B0034)</li> |
| | + | </ul> |
| | + | </p> |
| | + | |
| | | | |
| | | | |
| Line 312: |
Line 477: |
| | | | |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| - | <!-- -pTet description-- --> | + | <!-- --pLux description- --> |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| | | | |
| - | <a name='pTet'></a><h2>pTet promoter</h2>
| |
| | | | |
| - | <ol>
| + | |
| - | <ul> | + | <a name='pLux'></a><h2>p<sub>Lux</sub> promoter</h2> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516230 "> BBa_K516230 </a> (wiki name: E21 ) pTet-RBS30-mRFP-TT </li>
| + | |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516231 "> BBa_K516231 </a> (wiki name: E22 ) pTet-RBS31-mRFP-TT </li>
| + | |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> (wiki name: E23 ) pTet-RBS32-mRFP-TT | + | |
| - | </li>
| + | |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_I13521 "> BBa_I13521 </a> pTet-RBS34-mRFP-TT
| + | |
| - | </li></ul>
| + | |
| - | </ul>
| + | |
| - | </ol> | + | |
| | <p align='justify'> | | <p align='justify'> |
| | | | |
| - | The protocols for the characterization of pTet promoter are reported in the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#pTet_protocol'>pTet measurement section</a>. <br>
| |
| - | This promoter is widely studied and characterized usually using the strong RBS BBa_B0034. Here we have characterized its transcriptional strength as a function of aTc induction (ng/ul) for different RBSs. Four different induction curves were obtained and are reported in figure:<br><br>
| |
| | | | |
| | + | </p> |
| | + | <ol><ul> |
| | | | |
| | | | |
| - | <table width='100%'><tr><td width='50%'> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516330 "> BBa_K516330 </a> (wiki name: E17 ) pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS30-mRFP-TT </li> |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E32_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/a/af/E32_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div>
| + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516331 "> BBa_K516331 </a> (wiki name: E18 ) pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS31-mRFP-TT </li> |
| - | </td> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516332 "> BBa_K516332 </a> (wiki name: E19 ) pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS32-mRFP-TT </li> |
| - | <td width='50%'> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516334 "> BBa_K516334 </a> (wiki name: E20 ) pLambda-RBS30-LuxR-T-p<sub>Lux</sub>-RBS34-mRFP-TT </li></ul> |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E33_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/9/93/E33_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> | + | </ol> |
| - | </td></tr> | + | |
| - | <tr><td width='50%'> | + | |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E34_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/9/99/E34_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div>
| + | |
| - | </td> | + | |
| - | <td width='50%'> | + | |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E35_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/e/e4/E35_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> | + | |
| - | </td></tr> | + | |
| - | </table> | + | |
| | | | |
| - | The data collected from the mRFP measurement systems were processed as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements'> data analysis section</a>. The induction curves were obtained by fitting a Hill function as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Ptet_&_Plux'>modelling section</a> and the estimated <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Table_of_parameters'>parameters</a> for pTet are reported in the pictures and in table below. </p>
| |
| | <p align='justify'> | | <p align='justify'> |
| - | The estimated parameters of the Hill curves described in the figures are summarized in the table below: | + | The estimated parameters for the Hill functions are summarized in the table below. For more details on parameter estimation, see the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Ptet_&_Plux'>model section</a>. |
| | </p> | | </p> |
| - | <br><br>
| + | |
| - | <table class='data' width='100%' title='parameter value'> | + | <table class='data' width='100%'> |
| | <tr> | | <tr> |
| | <td class="row"><b>RBS</b></td> | | <td class="row"><b>RBS</b></td> |
| - | <td class="row"><b>α<sub>p<sub>Tet</sub></sub> [(AUr/min)/cell]</b></td> | + | <td class="row"><b>α<sub>p<sub>Lux</sub></sub> [(AUr/min)/cell]</b></td> |
| - | <td class="row"><b>δ<sub>p<sub>Tet</sub></sub> [-]</b></td> | + | <td class="row"><b>δ<sub>p<sub>Lux</sub></sub> [-]</b></td> |
| - | <td class="row"><b>η<sub>p<sub>Tet</sub></sub> [-]</b></td> | + | <td class="row"><b>η<sub>p<sub>Lux</sub></sub> [-]</b></td> |
| - | <td class="row"><b>k<sub>p<sub>Tet</sub></sub> [nM]</b></td> | + | <td class="row"><b>k<sub>p<sub>Lux</sub></sub> [ng/ml]</b></td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0030</td> | | <tr><td class="row">BBa_B0030</td> |
| - | <td class="row">230.67 [3.7]</td> | + | <td class="row">438 [10]</td> |
| - | <td class="row">0.028 [91.61]</td> | + | <td class="row">0.05 [>100]</td> |
| - | <td class="row">4.61 [23.73]</td> | + | <td class="row">2 [47]</td> |
| - | <td class="row">8.75 [4.16]</td> | + | <td class="row">1.88 [27]</td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0031</td> | | <tr><td class="row">BBa_B0031</td> |
| - | <td class="row">ND</td> | + | <td class="row">9.8 [7]</td> |
| - | <td class="row">ND</td> | + | <td class="row">0.11 [57]</td> |
| - | <td class="row">ND</td> | + | <td class="row">1.2 [29]</td> |
| - | <td class="row">ND</td> | + | <td class="row">1.5 [26]</td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0032</td> | | <tr><td class="row">BBa_B0032</td> |
| - | <td class="row">55.77 [12]</td> | + | <td class="row">206 [3]</td> |
| - | <td class="row">1.53E-11 [>>100]</td> | + | <td class="row">0 [>>100]</td> |
| - | <td class="row">4.98 [57.62]</td> | + | <td class="row">1.36 [10]</td> |
| - | <td class="row">7.26 [14.98]</td> | + | <td class="row">1.87 [9]</td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0034</td> | | <tr><td class="row">BBa_B0034</td> |
| - | <td class="row">120 [5.95]</td> | + | <td class="row">1105 [6]</td> |
| - | <td class="row">0.085 [40.6]</td> | + | <td class="row">0.02 [>100]</td> |
| - | <td class="row">24.85 [47.6]</td> | + | <td class="row">1.33 [19]</td> |
| - | <td class="row">9 [5.43]</td> | + | <td class="row">2.34 [18]</td> |
| | </tr> | | </tr> |
| | </table> | | </table> |
| - | Data are provided as average [CV%] | + | <div align="center">Data are provided as average [CV%].</div><br> |
| - | <br><br> | + | |
| | | | |
| - | <p align='justify'>
| |
| - | The measurement system pTet-B0031-mRFP-TT couldn't be assayed because its fluorescence output is under the detectability threshold of our measurement instrument. For this reason, the parameters of the corresponding Hill curve couldn't be estimated and are reported as 'Not Determined' ND.
| |
| - | </p>
| |
| | | | |
| - | <p align='justify'> | + | <div align="justify"> |
| - | α parameter (representing the maximum trascriptional rate in the studied range of induction) varies as expected with the RBS variation and also the δ and η parameters are quite different among the RBS variations.<br> | + | <p>From this table, it is evident that, whilst α<sub>p<sub>Lux</sub></sub> assumes significantly different values for different RBSs, η<sub>pLux</sub> and k<sub>pLux</sub> assume very similar values. This result shows that RBS variation modulates the amplitude of Hill function, not affecting the shape of the curve. The four induction curves result to be the same Hill function modulated in amplitude by a parameter, that is the estimated RBS efficiency for this measurement system.</p> |
| - | The K<sub>pTet</sub> parameter is quite constant among the RBS variations, thus suggesting that in this case the RBS variation doesn't substantially affect the switch point of the Hill curve, even if the amplitude and the maximum slope are not quite maintained (for the η parameter, maybe fitting problems).
| + | <p>These results are quite encouraging, because suggest that, given the non-modular behavior of RBS dpending on the encoded gene, the RBS has a modular behaviour respect to the promoter.</p> |
| - | | + | <p>The operative parameters are summarized in the table below:</p></div> |
| - | </p> | + | |
| - | <p align='justify'> | + | |
| - | The operative parameters are summarized in the table below: | + | |
| - | </p> | + | |
| | | | |
| | <table align='center' class='data' width='100%'> | | <table align='center' class='data' width='100%'> |
| Line 407: |
Line 546: |
| | <td class='row'><b>RPU<sub>max</sub></b></td> | | <td class='row'><b>RPU<sub>max</sub></b></td> |
| | <td class='row'><b>RPU<sub>min</sub></b></td> | | <td class='row'><b>RPU<sub>min</sub></b></td> |
| - | <td class='row'><b>Switch point [ng/ml]</b></td> | + | <td class='row'><b>Switch point [nM]</b></td> |
| - | <td class='row'><b>Linear boundaries [MIN; MAX] [ng/ml]</b></td> | + | <td class='row'><b>Linear boundaries [MIN; MAX] [nM]</b></td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0030</td><td class='row'>1.53</td><td class='row'>~0</td><td class='row'>7.95</td><td class='row'>[4.66;11.99]</td> | + | <td class='row'>B0030</td><td class='row'>4.28</td><td class='row'>0.20</td><td class='row'>1.08</td><td class='row'>[0.36; 3.27]</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0031</td><td class='row'>ND</td><td class='row'>ND</td><td class='row'>ND</td><td class='row'>ND</td> | + | <td class='row'>B0031</td><td class='row'>4.93</td><td class='row'>0.55</td><td class='row'>0.25</td><td class='row'>[0.03; 2.30]</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0032</td><td class='row'>3.16</td><td class='row'>~0</td><td class='row'>6.7</td><td class='row'>[4.45;10.05]</td> | + | <td class='row'>B0032</td><td class='row'>9.49</td><td class='row'>0.02</td><td class='row'>0.47</td><td class='row'>[0.07; 3.07]</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0034</td><td class='row'>2.73</td><td class='row'>0.23</td><td class='row'>8.96</td><td class='row'>[8.27;9.71]</td> | + | <td class='row'>B0034</td><td class='row'>21.53</td><td class='row'>0.51</td><td class='row'>0.53</td><td class='row'>[0.08; 3.77]</td> |
| | </tr> | | </tr> |
| | + | </table> |
| | + | <p align='justify'>These operative parameters provide useful information on the behavior of this 3OC6-HSL inducible device. RPU<sub>max</sub> assumes very different values in terms of RPUs. |
| | + | This can't be explained by RBS modulation, since RPUs have been evaluated by normalizing S<sub>cell</sub> of p<sub>Lux</sub>-RBSx for the one of J23101-RBSx. It is evident that some nonlinear effect on maximum strength, maybe due to saturation phenomena on protein expression, occur. |
| | + | The same RPUs should be observed for every RBS, since the normalization by the standard reference used for RPUs computation should eliminate the RBS contribute. Here different RPUs are observed, maybe due to nonlinear RBS behavior or to saturation phenomena occurring with this very strong promoter. The switch point and linear boundaries are quite constant in all the cases, showing that the linear region of this system is not affected by RBS changes.</p> |
| | + | |
| | + | <table width='100%'><tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/5/50/E17_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/5/50/E17_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/c/c4/E18_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/c/c4/E18_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | <tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/7/78/E19_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/7/78/E19_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/5/55/E20_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/5/55/E20_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | </table> | | </table> |
| | | | |
| - | <p align='justify'>
| |
| - | From these parameters, it is evident that whilst the switch-point is almost maintained for all the RBSs, the linear boundaries are similar for RBS30 and RBS32 but for RBS34 are moved on the right of one order of magnitude.
| |
| - | </p>
| |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| - |
| |
| | | | |
| | | | |
| Line 434: |
Line 586: |
| | | | |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| - | <!-- --pLux description- --> | + | <!-- -pTet description-- --> |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| | | | |
| | + | <a name='pTet'></a><h2>p<sub>Tet</sub> promoter</h2> |
| | | | |
| | + | <ol> |
| | + | <ul> |
| | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516230 "> BBa_K516230 </a> (wiki name: E21 ) p<sub>Tet</sub>-RBS30-mRFP-TT </li> |
| | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516231 "> BBa_K516231 </a> (wiki name: E22 ) p<sub>Tet</sub>-RBS31-mRFP-TT </li> |
| | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> (wiki name: E23 ) p<sub>Tet</sub>-RBS32-mRFP-TT |
| | + | </li> |
| | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_I13521 "> BBa_I13521 </a> p<sub>Tet</sub>-RBS34-mRFP-TT |
| | + | </li></ul> |
| | + | </ul> |
| | + | </ol> |
| | | | |
| - | <a name='pLux'></a><h2>pLux promoter</h2> | + | <div align="justify"> |
| - | <p align='justify'> | + | <p>The protocols for the characterization of p<sub>Tet</sub> promoter are reported in the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#pTet_protocol'>p<sub>Tet</sub> measurement section</a>.</p> |
| | + | <p>The data collected from the mRFP measurement systems were processed as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements'> data analysis section</a>. The induction curves were obtained by fitting a Hill function as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Ptet_&_Plux'>modelling section</a> and the estimated <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Table_of_parameters'>parameters</a> for p<sub>Tet</sub> are reported in the pictures and in table below. </p> |
| | + | <p>This promoter is widely studied and characterized usually using the strong RBS BBa_B0034. Here we have characterized its transcriptional strength as a function of aTc induction (ng/ml) for different RBSs. Three different induction curves were obtained and are reported in figure:</p></div> |
| | | | |
| | + | <center><a href="https://static.igem.org/mediawiki/2011/a/af/E32_RPU_80.jpg" class="image"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/a/af/E32_RPU_80.jpg" class="thumbimage" width="50%"></a> |
| | + | </center> |
| | | | |
| - | </p>
| |
| - | <ol><ul>
| |
| | | | |
| | + | <!-- <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/9/93/E33_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/9/93/E33_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> --> |
| | + | |
| | + | <table width='100%'> |
| | + | <tr><td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/9/99/E34_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/9/99/E34_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="https://static.igem.org/mediawiki/2011/e/e4/E35_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/e/e4/E35_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | </table> |
| | | | |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516330 "> BBa_K516330 </a> (wiki name: E17 ) pLambda-RBS30-LuxR-T-pLux-RBS30-mRFP-TT </li>
| |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516331 "> BBa_K516331 </a> (wiki name: E18 ) pLambda-RBS30-LuxR-T-pLux-RBS31-mRFP-TT </li>
| |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516332 "> BBa_K516332 </a> (wiki name: E19 ) pLambda-RBS30-LuxR-T-pLux-RBS32-mRFP-TT </li>
| |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516334 "> BBa_K516334 </a> (wiki name: E20 ) pLambda-RBS30-LuxR-T-pLux-RBS34-mRFP-TT </li></ul>
| |
| - | </ol>
| |
| | | | |
| | <p align='justify'> | | <p align='justify'> |
| - | The estimated parameters for the Hill functions are summarized in the table below. For more details on parameter estimation, see the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Ptet_&_Plux'>model section</a>. | + | The estimated parameters of the Hill curves described in the figures are summarized in the table below: |
| | </p> | | </p> |
| - | | + | <br> |
| - | <table class='data' width='100%'> | + | <table class='data' width='100%' title='parameter value'> |
| | <tr> | | <tr> |
| | <td class="row"><b>RBS</b></td> | | <td class="row"><b>RBS</b></td> |
| - | <td class="row"><b>α<sub>p<sub>Lux</sub></sub> [(AUr/min)/cell]</b></td> | + | <td class="row"><b>α<sub>p<sub>Tet</sub></sub> [(AUr/min)/cell]</b></td> |
| - | <td class="row"><b>δ<sub>p<sub>Lux</sub></sub> [-]</b></td> | + | <td class="row"><b>δ<sub>p<sub>Tet</sub></sub> [-]</b></td> |
| - | <td class="row"><b>η<sub>p<sub>Lux</sub></sub> [-]</b></td> | + | <td class="row"><b>η<sub>p<sub>Tet</sub></sub> [-]</b></td> |
| - | <td class="row"><b>k<sub>p<sub>Lux</sub></sub> [ng/ml]</b></td> | + | <td class="row"><b>k<sub>p<sub>Tet</sub></sub> [nM]</b></td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0030</td> | | <tr><td class="row">BBa_B0030</td> |
| - | <td class="row">438 [10]</td> | + | <td class="row">230.67 [3.7]</td> |
| - | <td class="row">0.05 [>100]</td> | + | <td class="row">0.028 [91.61]</td> |
| - | <td class="row">2 [47]</td> | + | <td class="row">4.61 [23.73]</td> |
| - | <td class="row">1.88 [27]</td> | + | <td class="row">8.75 [4.16]</td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0031</td> | | <tr><td class="row">BBa_B0031</td> |
| - | <td class="row">9.8 [7]</td> | + | <td class="row">ND</td> |
| - | <td class="row">0.11 [57]</td> | + | <td class="row">ND</td> |
| - | <td class="row">1.2 [29]</td> | + | <td class="row">ND</td> |
| - | <td class="row">1.5 [26]</td> | + | <td class="row">ND</td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0032</td> | | <tr><td class="row">BBa_B0032</td> |
| - | <td class="row">206 [3]</td> | + | <td class="row">55.77 [12]</td> |
| - | <td class="row">0 [>>100]</td> | + | <td class="row">1.53E-11 [>>100]</td> |
| - | <td class="row">1.36 [10]</td> | + | <td class="row">4.98 [57.62]</td> |
| - | <td class="row">1.87 [9]</td> | + | <td class="row">7.26 [14.98]</td> |
| | </tr> | | </tr> |
| | <tr><td class="row">BBa_B0034</td> | | <tr><td class="row">BBa_B0034</td> |
| - | <td class="row">1105 [6]</td> | + | <td class="row">120 [5.95]</td> |
| - | <td class="row">0.02 [>100]</td> | + | <td class="row">0.085 [40.6]</td> |
| - | <td class="row">1.33 [19]</td> | + | <td class="row">24.85 [47.6]</td> |
| - | <td class="row">2.34 [18]</td> | + | <td class="row">9 [5.43]</td> |
| | </tr> | | </tr> |
| | </table> | | </table> |
| | Data are provided as average [CV%] | | Data are provided as average [CV%] |
| - | | + | <br><br> |
| | | | |
| | <p align='justify'> | | <p align='justify'> |
| - | From this table, it is evident that, whilst α<sub>pLux</sub> assumes significantly different values for different RBSs, η<sub>pLux</sub> and k<sub>pLux</sub> assume very similar values. This result shows that RBS variation modulates the amplitude of Hill function, not affecting the shape of the curve. The four induction curves result to be the same Hill function modulated in amplitude by a parameter, that is the estimated RBS efficiency for this measurement system.<br>
| + | The measurement system p<sub>Tet</sub>-B0031-mRFP-TT couldn't be assayed because its fluorescence output is under the detectability threshold of our measurement instrument. For this reason, the parameters of the corresponding Hill curve couldn't be estimated and are reported as 'Not Determined' ND. |
| - | These results are quite encouraging, because suggest that, given the non-modular behavior of RBS dpending on the encoded gene, the RBS has a modular behaviour respect to the promoter.
| + | |
| - | | + | |
| | </p> | | </p> |
| | | | |
| | + | <div align='justify'> |
| | + | <p>α parameter (representing the maximum trascriptional rate in the studied range of induction) varies as expected with the RBS variation and also the δ and η parameters are quite different among the RBS variations.</p> |
| | + | <p>The k<sub>p<sub>Tet</sub></sub> parameter is quite constant among the RBS variations, thus suggesting that in this case the RBS variation doesn't substantially affect the switch point of the Hill curve, even if the amplitude and the maximum slope are not quite maintained (for the η parameter, maybe fitting problems). |
| | + | |
| | + | </p> |
| | <p align='justify'> | | <p align='justify'> |
| | The operative parameters are summarized in the table below: | | The operative parameters are summarized in the table below: |
| - | </p> | + | </p></div> |
| | | | |
| | <table align='center' class='data' width='100%'> | | <table align='center' class='data' width='100%'> |
| Line 508: |
Line 684: |
| | <td class='row'><b>RPU<sub>max</sub></b></td> | | <td class='row'><b>RPU<sub>max</sub></b></td> |
| | <td class='row'><b>RPU<sub>min</sub></b></td> | | <td class='row'><b>RPU<sub>min</sub></b></td> |
| - | <td class='row'><b>Switch point [nM]</b></td> | + | <td class='row'><b>Switch point [ng/ml]</b></td> |
| - | <td class='row'><b>Linear boundaries [MIN; MAX] [nM]</b></td> | + | <td class='row'><b>Linear boundaries [MIN; MAX] [ng/ml]</b></td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0030</td><td class='row'>4.28</td><td class='row'>0.20</td><td class='row'>1.08</td><td class='row'>[0.36; 3.27]</td> | + | <td class='row'>B0030</td><td class='row'>1.53</td><td class='row'>~0</td><td class='row'>7.95</td><td class='row'>[4.66;11.99]</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0031</td><td class='row'>4.93</td><td class='row'>0.55</td><td class='row'>0.25</td><td class='row'>[0.03; 2.30]</td> | + | <td class='row'>B0031</td><td class='row'>ND</td><td class='row'>ND</td><td class='row'>ND</td><td class='row'>ND</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0032</td><td class='row'>9.49</td><td class='row'>0.02</td><td class='row'>0.47</td><td class='row'>[0.07; 3.07]</td> | + | <td class='row'>B0032</td><td class='row'>3.16</td><td class='row'>~0</td><td class='row'>6.7</td><td class='row'>[4.45;10.05]</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class='row'>B0034</td><td class='row'>21.53</td><td class='row'>0.51</td><td class='row'>0.53</td><td class='row'>[0.08; 3.77]</td> | + | <td class='row'>B0034</td><td class='row'>2.73</td><td class='row'>0.23</td><td class='row'>8.96</td><td class='row'>[8.27;9.71]</td> |
| | </tr> | | </tr> |
| | </table> | | </table> |
| | + | |
| | <p align='justify'> | | <p align='justify'> |
| | + | From these parameters, it is evident that whilst the switch-point is almost maintained for all the RBSs, the linear boundaries are similar for RBS30 and RBS32 but for RBS34 are moved on the right of one order of magnitude. |
| | + | </p> |
| | + | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| | | | |
| - | These operative parameters provide useful information on the behavior of this 3OC6-HSL inducible device. RPU<sub>max</sub> assumes very different values in terms of RPUs.
| |
| - | This can't be explained by RBS modulation, since RPUs have been evaluated by normalizing S<sub>cell</sub> of pLux-RBSx for the one of J23101-RBSx. It is evident that some nonlinear effect, maybe due to non-modularity of RBS when the promoter changes or maybe due to saturation effects on protein expression, occur. The switch point are almost constant
| |
| | | | |
| | | | |
| - | showing that the modulation in amplitude of the Hill can't be explained by a linear dependance on the RBS efficiency (in this case, in fact, the same RPUs should be observed for every RBS, since the standard reference used for RPUs computation).
| |
| | | | |
| - | </p>
| |
| | | | |
| - | <table width='100%'><tr><td width='50%'>
| |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E17_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/5/50/E17_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div>
| |
| - | </td>
| |
| - | <td width='50%'>
| |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E18_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/c/c4/E18_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div>
| |
| - | </td></tr>
| |
| - | <tr><td width='50%'>
| |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E19_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/7/78/E19_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div>
| |
| - | </td>
| |
| - | <td width='50%'>
| |
| - | <div style="text-align:justify"><div class="thumbinner" width='100%'><a href="File:E20_RPU_80.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/5/55/E20_RPU_80.jpg" class="thumbimage" width="100%"></a></div></div>
| |
| - | </td></tr>
| |
| - | </table>
| |
| - |
| |
| - | <div align="right"><small><a href="#indice" title="">^top</a></small></div>
| |
| | | | |
| | | | |
| Line 562: |
Line 723: |
| | <p align='justify'> | | <p align='justify'> |
| | | | |
| - | LuxI has been characterized through the Biosensor BBa_T9002 (see <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#introduction_to_T9002'>modelling section</a>). | + | LuxI has been characterized through the Biosensor BBa_T9002 (see <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#introduction_to_T9002'>modelling section</a>) using the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetLuxI'>p<sub>Tet</sub>-RBSx-LuxI-TT</a> measurement systems. |
| - | <br>The HSL synthesis rate has been evaluated according to the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>model equations</a>, properly adjusted. In particular, the ODE system is reported here: | + | <br>The HSL synthesis rate has been evaluated according to the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>model equations</a>, properly adjusted. In particular, the ODE system is reported here: </p> |
| | + | |
| | + | <div style='text-align:justify'><div class="thumbinner" style="width: 850px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2011/a/a6/Pc_luxi.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/a/a6/Pc_luxi.jpg" class="thumbimage" height="70%" width="68%"></a></div></div> |
| | | | |
| - | <div style="text-align:justify"><div class="thumbinner" width='80%'><a href="https://static.igem.org/mediawiki/2011/a/a6/Pc_luxi.jpg" class="image"><img alt="System of equations for LuxI characterization" src="https://static.igem.org/mediawiki/2011/e/ec/UNIPV_caratt_PLux.jpg" width="55%"></a></div></div>
| |
| | | | |
| | | | |
| | <p align='justify'> | | <p align='justify'> |
| | + | Since the measurement systems are only assayed in the exponential growth phase, in the equation (3) N < <N<sub>max</sub> is assumed. |
| | + | The parameters N<sub>max</sub>, μ and γ<sub>HSL</sub> (see the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Results'>Results section</a> for more details) are known and summarized in the table below:</p> |
| | | | |
| - | Since the measurement systems are only assayed in the exponential growth phase, the third equation can be modified as follows:
| + | <div align="center"> |
| - | </p>
| + | <table align="center" class='data' title='parameter value'> |
| - | dN/dt=N*μ
| + | |
| - | | + | |
| - | <p align='justify'>
| + | |
| - | because N<N<sub>max</sub>. <br>
| + | |
| - | The parameters N<sub>max</sub>, μ and γ<sub>HSL</sub> (see the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Results'>Results section</a> for more details) are known. <br> These parameters are summarized in the table below:</p>
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | <table class='data' width='100%' title='parameter value'>
| + | |
| | <tr> | | <tr> |
| | <td class="row"><b>N<sub>max</sub></sub></b></td> | | <td class="row"><b>N<sub>max</sub></sub></b></td> |
| Line 588: |
Line 745: |
| | <tr> | | <tr> |
| | <td class='row' >1*10<sup>9</sup></td> | | <td class='row' >1*10<sup>9</sup></td> |
| - | <td class='row' >0.003152</td> | + | <td class='row' >0.004925</td> |
| | <td class='row' >0</td> | | <td class='row' >0</td> |
| | </table> | | </table> |
| | </p> | | </p> |
| - | | + | </div> |
| | | | |
| | <p align='justify'> | | <p align='justify'> |
| - | The parameters V<sub>max</sub>, K<sub>M,LuxI</sub> and α<sub>RBSx</sub> were estimated with a simultaneous fitting of the data collected as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#LuxI'>measurement section</a> for the four measurement parts <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetLuxI'>pTet-RBSx-LuxI-TT</a> assayed by <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#T9002'>BBa_T9002 biosensor</a> section. | + | The parameters V<sub>max</sub>, k<sub>M,LuxI</sub> and α<sub>RBSx</sub> were estimated with a simultaneous fitting of the data collected as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#LuxI'>measurement section</a> for the four measurement parts <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetLuxI'>p<sub>Tet</sub>-RBSx-LuxI-TT</a> assayed by <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#T9002'>BBa_T9002 biosensor</a> section. |
| | + | |
| | | | |
| - | The estimated parameters for the enzymatic activity of LuxI are reported in the table below:
| + | <div align="justify"><div class="thumbinner" style="width: 850px;"> |
| | + | <a href="https://static.igem.org/mediawiki/2011/4/40/Luxi_prod.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/4/40/Luxi_prod.jpg" class="thumbimage" width="85%" height="50%"></a></div></div> |
| | | | |
| - | <table align='center' width='50%'> | + | |
| - | <tr><td width='100%'> | + | <div align="justify"><p>The estimated parameters for the enzymatic activity of LuxI are reported in the table below:</p></div> |
| - | <table class='data' width='100%'> | + | |
| | + | |
| | + | <div align=center"> |
| | + | <table align="center" class='data'> |
| | <tr> | | <tr> |
| - | <td class='row'><b>K<sub>M, LuxI</sub></b></td>
| |
| | <td class='row'><b>V<sub>max</sub></b></td> | | <td class='row'><b>V<sub>max</sub></b></td> |
| | + | <td class='row'><b>k<sub>M,LuxI</sub></b></td> |
| | <td class='row'><b>α<sub>B0030</sub></b></td> | | <td class='row'><b>α<sub>B0030</sub></b></td> |
| | <td class='row'><b>α<sub>B0031</sub></b></td> | | <td class='row'><b>α<sub>B0031</sub></b></td> |
| Line 610: |
Line 773: |
| | <td class='row'><b>α<sub>B0034</sub></b></td> | | <td class='row'><b>α<sub>B0034</sub></b></td> |
| | </tr> | | </tr> |
| - | <tr><td class='row'>1.5*10<sup>4</sup></td> | + | <tr><td class='row'>3.56*10<sup>-9</sup></td> |
| - | <td class='row'>3.63*10<sup>-9</sup></td> | + | <td class='row'>6.87*10<sup>3</sup></td> |
| - | <td class='row'>247</td> | + | <td class='row'> 87 </td> |
| - | <td class='row'>15</td> | + | <td class='row'>8.5</td> |
| | <td class='row'>ND</td> | | <td class='row'>ND</td> |
| - | <td class='row'>553</td> | + | <td class='row'> 252 </td> |
| - | | + | </tr></table> |
| - | </tr></table></td></tr></table> | + | </div> |
| - | | + | <br> |
| - | <br><br> | + | |
| - |
| + | |
| | </p> | | </p> |
| | + | </p> |
| | + | |
| | + | <p align='justify'> |
| | + | The provided parameters k<sub>M</sub> and V<sub>max</sub> represent the enzymatic activity of LuxI, described by our model. They must not be confused with the operative parameters of the Michaelis-Menten relation. |
| | + | These synthetic parameters have a great importance, since they can be used in more complicated models in order to predict the behavior of complex circuits. |
| | </p> | | </p> |
| | | | |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| | + | |
| | | | |
| | | | |
| Line 634: |
Line 801: |
| | <!-- --AiiA description-- --> | | <!-- --AiiA description-- --> |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| - |
| |
| | | | |
| | | | |
| Line 640: |
Line 806: |
| | | | |
| | <p align='justify'> | | <p align='justify'> |
| - | The activity of AiiA enzyme has been evaluated by testing the measurement systems <a href="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg">pTet-RBSx-AiiA-TT</a>. | + | The activity of AiiA enzyme has been evaluated by testing the measurement systems <a href="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg">p<sub>Tet</sub>-RBSx-AiiA-TT</a>. |
| - | The HSL synthesis rate has been evaluated according to the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>model equations</a>, properly adjusted. In particular, the ODE system is reported here:
| + | Similar to LuxI, a system of differential equations (referring to <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Modelling#Equations_for_gene_networks'>model equations</a>) has been derived. |
| | </p> | | </p> |
| | | | |
| - | | + | <div style='text-align:justify'> |
| - | <div style="text-align:justify"><div class="thumbinner" width='80%'><a href="File:Eq characterized2.jpg" class="image"><img alt="System of equations for AiiA characterization" src="https://static.igem.org/mediawiki/2011/a/a0/Eq_characterized2.jpg" class="thumbimage" width="55%"></a></div></div> | + | <div class="thumbinner" style="width: 850px;"> |
| - | | + | <a href="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg" class="thumbimage" height="70%" width="68%"></a> |
| | + | </div></div> |
| | | | |
| | <p align='justify'> | | <p align='justify'> |
| | + | The parameters k<sub>cat</sub>, k<sub>M,AiiA</sub> and α<sub>RBSx</sub> would have been estimated with a simultaneous fitting of the data collected as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#AiiA'>measurement section</a> for the four measurement parts <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetAiiA'>p<sub>Tet</sub>-RBSx-AiiA-TT</a> assayed by <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#T9002'>BBa_T9002 biosensor</a> section. |
| | + | Unfortunately, their estimation revealed difficult.<br> |
| | + | In the first experiments with the measurement system <a href="https://static.igem.org/mediawiki/2011/1/11/Pc_aiia.jpg">p<sub>Tet</sub>-RBSx-AiiA-TT</a> in LOW-COPY at pH=7 no degradation of HSL was observed. The collected data are shown in the figure below. HSL degradation is identical in the measurement system and in the negative control after 21 hours. |
| | | | |
| - | Since the measurement systems are only assayed in the exponential growth phase, the third equation can be modified as follows:
| |
| - | </p>
| |
| - | dN/dt=N*μ
| |
| | | | |
| - | <p align='justify'>
| + | <table width='100%'><tr><td width='50%'> |
| - | because N<N<sub>max</sub>. <br>
| + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | The parameters N<sub>max</sub>, μ and γ<sub>HSL</sub> (see the <a href='https://2011.igem.org/Team:UNIPV-Pavia/Project/Results'>Results section</a> for more details) are known. <br> These parameters are summarized in the table below:
| + | <a href="https://static.igem.org/mediawiki/2011/6/69/Aiia_LC_B0030.jpg"> |
| - | | + | <img alt="" src="https://static.igem.org/mediawiki/2011/6/69/Aiia_LC_B0030.jpg" class="thumbimage" width="100%"></a></div></div> |
| - | <table class='data' width='100%' title='parameter value'> | + | </td> |
| - | <tr> | + | <td width='50%'> |
| - | <td class="row"><b>N<sub>max</sub></sub></b></td> | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | <td class="row"><b>μ</b></td> | + | <a href="https://static.igem.org/mediawiki/2011/6/63/Aiia_LC_B0031.jpg"> |
| - | <td class="row"><b>γ<sub>HSL</sub></b></td> | + | <img alt="" src="https://static.igem.org/mediawiki/2011/6/63/Aiia_LC_B0031.jpg" class="thumbimage" width="100%"></a></div></div> |
| - | </tr> | + | </td></tr> |
| - | <tr> | + | <tr><td width='50%'> |
| - | <td class='row' >1*10<sup>9</sup></td> | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | <td class='row' >0.003152</td> | + | <a href="https://static.igem.org/mediawiki/2011/f/f5/Aiia_LC_B0032.jpg"> |
| - | <td class='row' >0</td> | + | <img alt="" src="https://static.igem.org/mediawiki/2011/f/f5/Aiia_LC_B0032.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td> |
| | + | <td width='50%'> |
| | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| | + | <a href="https://static.igem.org/mediawiki/2011/8/8d/Aiia_LC_B0034.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/8/8d/Aiia_LC_B0034.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | </table> | | </table> |
| - | </p>
| |
| | | | |
| | | | |
| | | | |
| - | <p align='justify'>
| |
| | | | |
| - | The parameters K<sub>cat</sub>, K<sub>M,AiiA</sub> and α<sub>RBSx</sub> were estimated with a simultaneous fitting of the data collected as described in <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#AiiA'>measurement section</a> for the four measurement parts <a href='https://2011.igem.org/Team:UNIPV-Pavia/Parts/Characterized#pTetAiiA'>pTet-RBSx-AiiA-TT</a> assayed by <a href='https://2011.igem.org/Team:UNIPV-Pavia/Measurements#T9002'>BBa_T9002 biosensor</a> section.
| |
| | | | |
| - | The estimated parameters for the enzymatic activity of AiiA are reported in the table below:
| + | <div align="justify">Further investigation on the enzyme were performed in more suitable conditions, to evaluate its intrinsic activity. AiiA activity was investigated in HIGH COPY plasmid in <em>E. coli</em> TOP10, in order to understand if the enzyme worked. In this case, a significant difference in degradation between p<sub>Tet</sub>-RBSx-AiiA-TT and the negative control was observed, also just after 7 hours. That was the proof of the good functioning (in HIGH COPY) of the enzyme.</div> |
| | + | |
| | | | |
| - | <table align='center' width='50%'> | + | <table width='100%'><tr><td width='50%'> |
| - | <tr><td width='100%'> | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | <table class='data' width='100%'> | + | <a href="https://static.igem.org/mediawiki/2011/6/6c/Aiia_HC_B0030.jpg"> |
| - | <tr> | + | <img alt="" src="https://static.igem.org/mediawiki/2011/6/6c/Aiia_HC_B0030.jpg" class="thumbimage" width="100%"></a></div></div> |
| - | <td class='row'><b>K<sub>cat</sub></b></td> | + | </td> |
| - | <td class='row'><b>K<sub>M, AiiA</sub></b></td> | + | <td width='50%'> |
| - | <td class='row'><b>α<sub>B0030</sub></b></td> | + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | <td class='row'><b>α<sub>B0031</sub></b></td> | + | <a href="https://static.igem.org/mediawiki/2011/e/ec/Aiia_HC_B0031.jpg"> |
| - | <td class='row'><b>α<sub>B0032</sub></b></td> | + | <img alt="" src="https://static.igem.org/mediawiki/2011/e/ec/Aiia_HC_B0031.jpg" class="thumbimage" width="100%"></a></div></div> |
| - | <td class='row'><b>α<sub>B0034</sub></b></td>
| + | </td></tr> |
| - | </tr> | + | <tr><td width='50%'> |
| - | <tr><td class='row'></td>
| + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | <td class='row'></td> | + | <a href="https://static.igem.org/mediawiki/2011/b/b2/Aiia_HC_B0032.jpg"> |
| - | <td class='row'></td>
| + | <img alt="" src="https://static.igem.org/mediawiki/2011/b/b2/Aiia_HC_B0032.jpg" class="thumbimage" width="100%"></a></div></div> |
| - | <td class='row'></td> | + | </td> |
| - | <td class='row'></td> | + | <td width='50%'> |
| - | <td class='row'></td>
| + | <div style="text-align:justify"><div class="thumbinner" width='100%'> |
| - | </tr></table></td></tr></table> | + | <a href="https://static.igem.org/mediawiki/2011/d/d7/Aiia_HC_B0034.jpg"> |
| | + | <img alt="" src="https://static.igem.org/mediawiki/2011/d/d7/Aiia_HC_B0034.jpg" class="thumbimage" width="100%"></a></div></div> |
| | + | </td></tr> |
| | + | </table> |
| | | | |
| - | <br><br>
| |
| | | | |
| | + | <div align="justify"> |
| | + | Since the enzyme activity had been assessed, some suitable working conditions were investigated for AiiA. Since our studies on HSL stability for different pHs has shown that its stability improves at pH=6.0, this experimental condition was used to test once again MGZ1 strain with the system expressing AiiA in low copy number plasmid. |
| | + | |
| | + | Unfortunately, we had some problems with the biosensor, BBa_T9002, that didn't work as usual, resulting in a calibration curve modified. So, k<sub>M,AiiA</sub> and k<sub>cat</sub> were impossible to be estimated from these data. We decided to estimate them from experimental data coming from Tecan tests preformed in <em>E.COLI</em> TOP10, where aiiA gene was cloned in a high copy numebr plasmid, pSB1A2, downstream p<sub>Tet</sub> with different RBSs; in this way we could have some semi-quantitative information about the order of magnitude of these parameters. Taking into account the different copy number, we tried to simulate our model behavior with reasonable values for AiiA parameters.</div> |
| | + | <br> |
| | | | |
| | </p> | | </p> |
| - |
| |
| | | | |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| Line 715: |
Line 893: |
| | <!-- ----------------- --> | | <!-- ----------------- --> |
| | | | |
| - | <a name='K999'></a><h2>pSB1C3 plasmid with mRFP between S and P bearing pTet (easy-to-clone)</h2> | + | <a name='K999'></a><h2>pSB1C3 plasmid with mRFP between S and P bearing p<sub>Tet</sub> (easy-to-clone)</h2> |
| | <table align='center' width='100%'> | | <table align='center' width='100%'> |
| | <tr> | | <tr> |
| Line 724: |
Line 902: |
| | </tr> | | </tr> |
| | </table> | | </table> |
| - | This vector was designed and realized in order to facilitate the cloning of coding sequences downstream of the strong promoter pTet. This vector was assembled by ligating S-P excided mRFP coding sequence from BBa_J61002 and ligating it in BBa_R0040 cut with S and P. Thus, the resulting vector contains mRFP between S and P. pTet cn be easily excided (E-P) and moved in the desired vector (E-P) and then the desired coding sequence can be easily assembled by digesting S-P the vector and X-P the coding sequence, thus obtaining a final part that is standard10-compatible. | + | <div align="justify">This vector was designed and realized in order to facilitate the cloning of coding sequences downstream of the strong promoter p<sub>Tet</sub>. This vector was assembled by ligating S-P excided mRFP coding sequence from BBa_J61002 and ligating it in BBa_R0040 cut with S and P. Thus, the resulting vector contains mRFP between S and P. p<sub>Tet</sub> can be easily excided (E-P) and moved in the desired vector (E-P) and then the desired coding sequence can be easily assembled by digesting S-P the vector and X-P the coding sequence, thus obtaining a final part that is standard10-compatible.</div><br> |
| | | | |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |
| Line 730: |
Line 908: |
| | | | |
| | <a name='Improvement'></a><h1>Existing parts: sequence debugging</h1> | | <a name='Improvement'></a><h1>Existing parts: sequence debugging</h1> |
| | + | |
| | + | <div align="justify"> |
| | + | |
| | <ol><ul> | | <ol><ul> |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516021 "> BBa_K516021 </a> (wiki name: E10 ) RBS31-AiiA-TT Rebuilt existing part from BBa_I13914 (DNA planning) </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516021 "> BBa_K516021 </a> (wiki name: E10 ) RBS31-AiiA-TT Rebuilt existing part from BBa_I13914 (DNA planning) </li> |
| Line 735: |
Line 916: |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516030 "> BBa_K516030 </a> (wiki name: E5 ) RBS30-mRFP-TT Rebuilt existing part from BBa_S04180 (DNA planning) </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516030 "> BBa_K516030 </a> (wiki name: E5 ) RBS30-mRFP-TT Rebuilt existing part from BBa_S04180 (DNA planning) </li> |
| | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516032 "> BBa_K516032 </a> (wiki name: E7 ) RBS32-mRFP-TT Rebuilt existing part from BBa_ J133000 (DNA planning) </li> | | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516032 "> BBa_K516032 </a> (wiki name: E7 ) RBS32-mRFP-TT Rebuilt existing part from BBa_ J133000 (DNA planning) </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516214 "> BBa_K516214 </a> (wiki name: E16 ) pTet-RBS34-LuxI Rebuilt existing part from BBa_S03623 (DNA available, only 2008 kit, inconsistent) </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516214 "> BBa_K516214 </a> (wiki name: E16 ) p<sub>Tet</sub>-RBS34-LuxI Rebuilt existing part from BBa_S03623 (DNA available, only 2008 kit, inconsistent) </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516222 "> BBa_K516222 </a> (wiki name: E26 ) pTet-RBS32-AiiA-TT Rebuilt existing part from BBa_ J22071 (2008 only, Bad sequencing) </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516222 "> BBa_K516222 </a> (wiki name: E26 ) p<sub>Tet</sub>-RBS32-AiiA-TT Rebuilt existing part from BBa_ J22071 (2008 only, Bad sequencing) </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516224 "> BBa_K516224 </a> (wiki name: E27 ) pTet-RBS34-AiiA-TT Rebuilt existing part from BBa_K077047 (Part deleted) </li> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516224 "> BBa_K516224 </a> (wiki name: E27 ) p<sub>Tet</sub>-RBS34-AiiA-TT Rebuilt existing part from BBa_K077047 (Part deleted) </li> |
| - | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> (wiki name: E23 ) pTet-RBS32-mRFP-TT Rebuilt existing part from BBa_I20252 (DNA planning) </li></ul></ol> | + | <li> <A HREF="http://partsregistry.org/wiki/index.php/Part: BBa_K516232 "> BBa_K516232 </a> (wiki name: E23 ) p<sub>Tet</sub>-RBS32-mRFP-TT Rebuilt existing part from BBa_I20252 (DNA planning) </li></ul></ol> |
| | + | </div> |
| | | | |
| | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | | <div align="right"><small><a href="#indice" title="">^top</a></small></div> |

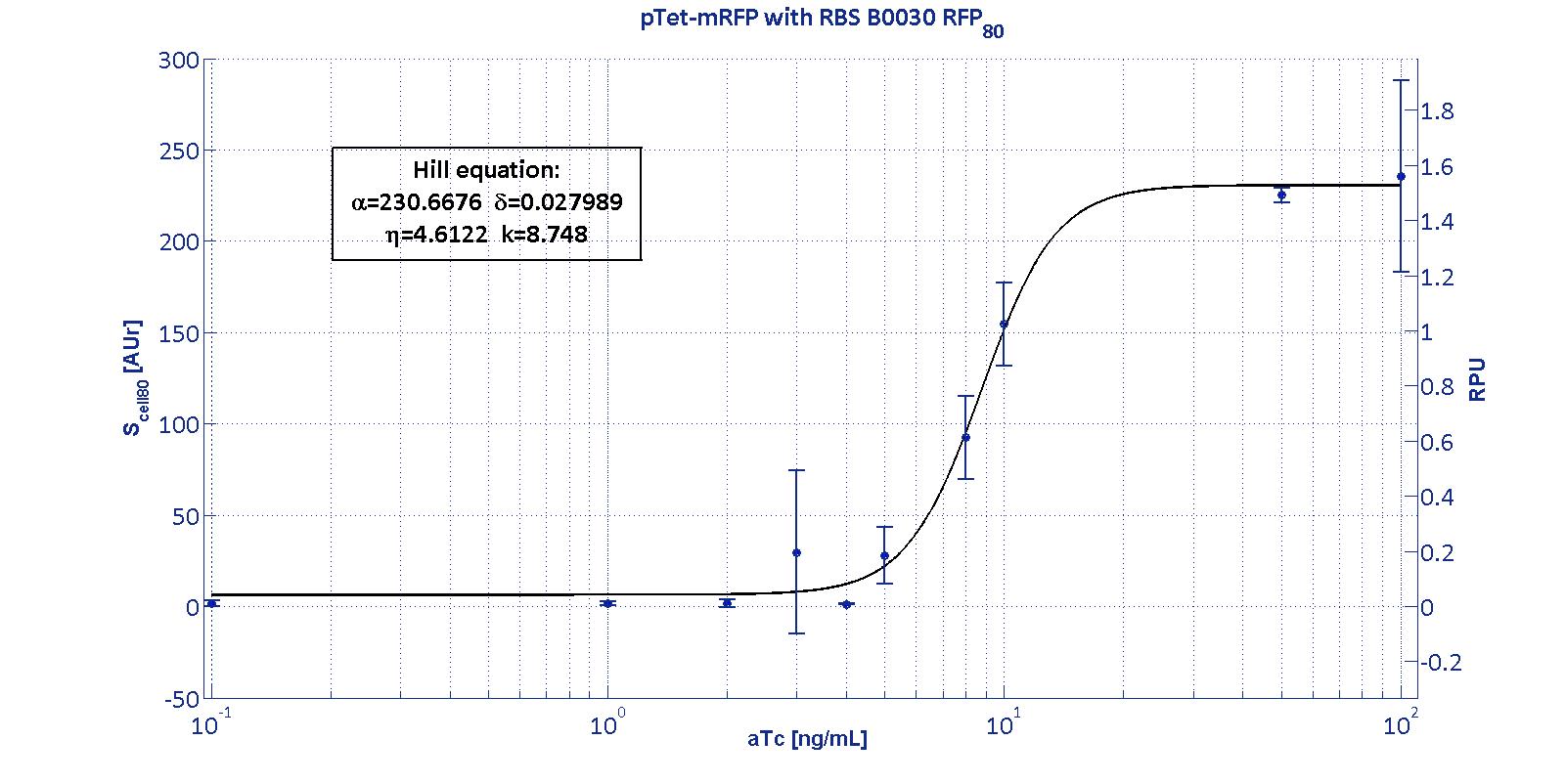

 "
"