Team:Bielefeld-Germany/Results
From 2011.igem.org
| Line 19: | Line 19: | ||
==Bisphenol A degradation with ''E. coli''== | ==Bisphenol A degradation with ''E. coli''== | ||
| - | The bisphenol A degradation with the BioBricks <partinfo>K123000</partinfo> and <partinfo>K123001</partinfo> works in ''E. coli'' KRX in general. Because Sasaki ''et al.'' (2008) reported problems with protein folding in ''E. coli'' which seem to avoid a complete BPA degradation, we did not use the strong T7 promoter for expressing these BioBricks but a medium strong constitutive promoter (<partinfo>J23110</partinfo>). With this promoter upstream of a polycistronic ''bisdAB'' gene we were able to completely degrade 120 mg L<sup>-1</sup> BPA in about 30 - 33 h. By fusing <partinfo>K123000</partinfo> and <partinfo>K123001</partinfo> together we could improve the BPA degradation of ''E. coli'' even further, so 120 mg L<sup>-1</sup> BPA can be degraded in 21 - 24 h. This data is shown in the following figure: | + | The bisphenol A degradation with the BioBricks <partinfo>K123000</partinfo> and <partinfo>K123001</partinfo> works in ''E. coli'' KRX in general. Because [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki ''et al.'' (2008)] reported problems with protein folding in ''E. coli'' which seem to avoid a complete BPA degradation, we did not use the strong T7 promoter for expressing these BioBricks but a medium strong constitutive promoter (<partinfo>J23110</partinfo>). With this promoter upstream of a polycistronic ''bisdAB'' gene we were able to completely degrade 120 mg L<sup>-1</sup> BPA in about 30 - 33 h. By fusing <partinfo>K123000</partinfo> and <partinfo>K123001</partinfo> together (using Freiburg BioBrick assembly standard) we could '''improve the BPA degradation''' of ''E. coli'' even further, so 120 mg L<sup>-1</sup> BPA can be degraded in 21 - 24 h. This data is shown in the following figure: |
| - | [[Image:Bielefeld-Germany2011_K123000K123001char.jpg|650px|thumb| '''Figure | + | [[Image:Bielefeld-Germany2011_K123000K123001char.jpg|650px|thumb| '''Figure 5: BPA degradation by ''E. coli'' KRX carrying genes for BisdA and BisdB (only ''bisdA'' (black), polycistronic ''bisdAB'' (red) and fusion protein between BisdA and BisdB (green)) behind the medium strong constitutive promoter <partinfo>J23110</partinfo> with RBS <partinfo>B0034</partinfo>. Cultivations were carried out at 30 °C in LB + Amp + BPA medium for 24 h and 36 h, respectively, with automatic sampling every three hours in 300 mL shaking flasks without baffles with silicon plugs. At least three biological replicates were analysed (three for ''bisdA'' alone, seven for ''bisdAB'' polycistronic and five for the fusion protein). ''']] |
| + | |||
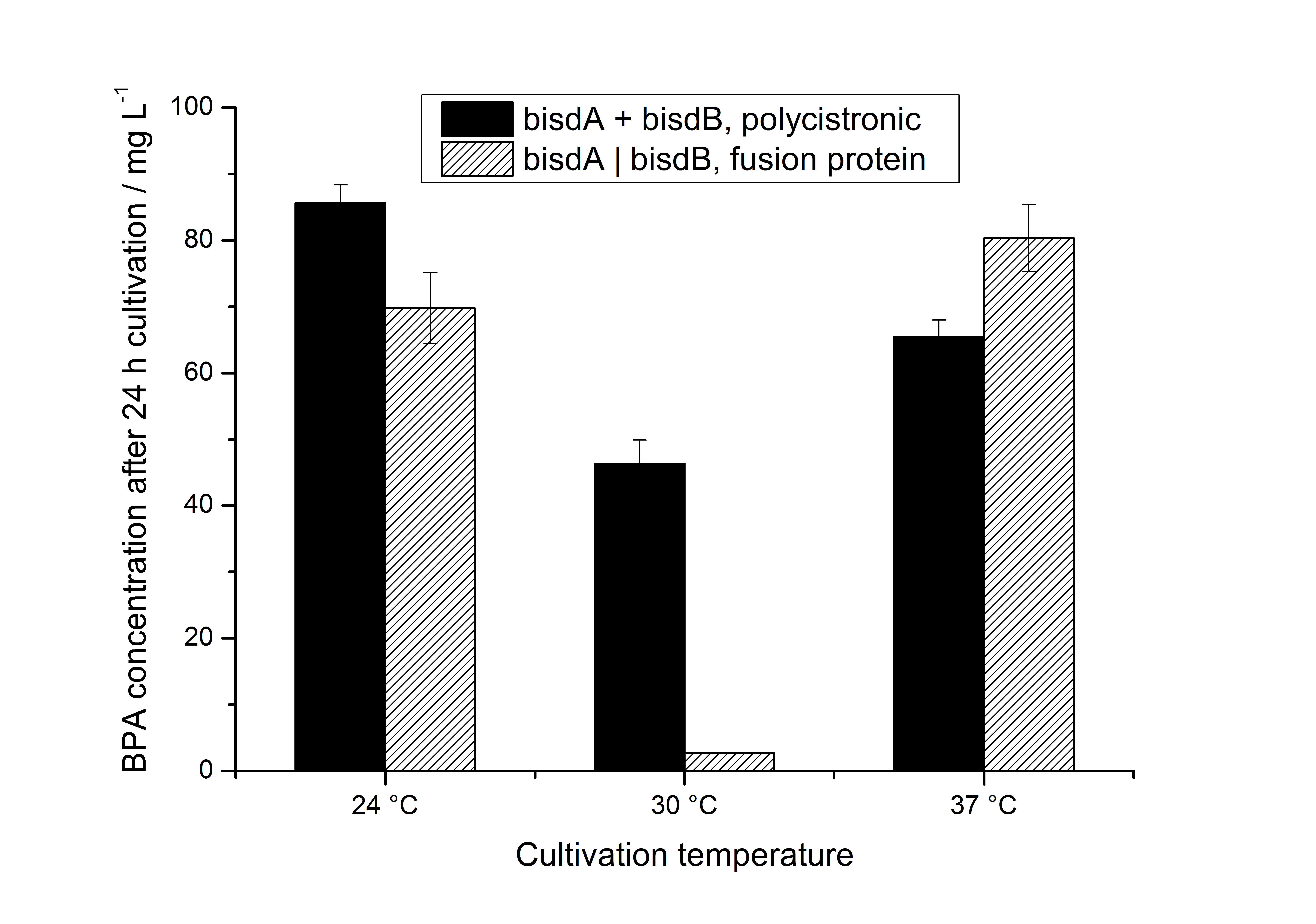
| + | We also carried out these cultivations at different temperatures and BPA concentrations, but the chosen conditions (30 °C and 120 mg L<sup>-1</sup> BPA) seem to be the best. Higher BPA concentrations have an effect on the growth of ''E. coli'' and higher temperature leeds to a worse BPA degradation (probably due to misfolding of the enzymes). These data on the effect of the temperature on the BPA degradation is shown in fig. 6. | ||
| + | |||
| + | [[Image:Bielefeld-Germany_temperatureBPAdegrad.jpg|650px|thumb| '''Figure 6: BPA degradation by ''E. coli'' KRX carrying genes for BisdA and BisdB (polycistronic ''bisdAB'' (black) and fusion protein between BisdA and BisdB (striped)) behind the medium strong constitutive promoter <partinfo>J23110</partinfo> with RBS <partinfo>B0034</partinfo>. Cultivations were carried out at different temperatures in LB + Amp + BPA medium (starting concentration 120 mg L<sup>-1</sup> BPA) for 24 h in 300 mL shaking flasks without baffles with silicon plugs. Samples were taken at the end of the cultivation. Three biological replicates were analysed. ''']] | ||
==Modelling of intracellular bisphenol A degradation== | ==Modelling of intracellular bisphenol A degradation== | ||
Revision as of 11:53, 17 September 2011

Contents |
Bisphenol A degradation
Sequencing results
The iGEM team from the University of Alberta sent in BioBricks for BPA degradation in 2008 (<partinfo>K123000</partinfo> and <partinfo>K123001</partinfo>). Sequencing of these BioBricks by the iGEM HQ and by us led to negative results so the sequences entered into the partsregistry are not correct although these sequences are the ones from Sphingomonas bisphenolicum AO1 (compare [http://www.ncbi.nlm.nih.gov/nuccore/AB255167.1 genbank entry]). In addition, there are "illegal" AgeI and NgoMIV restriction sites in the BioBrick which are used for Freiburg BioBrick assembly standard (RFC 25). After translating and comparing the original sequences from the partsregistry and the sequences from our sequencing results in silico we saw, that the amino acid sequences were identically. These BioBricks were probably synthesized in the Freiburg assembly standard 25 because they have the accordant restriction sites and they were codon optimized for Escherichia coli but the original sequence from S. bisphenolicum AO1 were entered into the registry because amino acid sequence of the real sequence and the sequence that was entered are identical. The alignments are shown in fig. 1 - 4.
Bisphenol A degradation with E. coli
The bisphenol A degradation with the BioBricks <partinfo>K123000</partinfo> and <partinfo>K123001</partinfo> works in E. coli KRX in general. Because [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki et al. (2008)] reported problems with protein folding in E. coli which seem to avoid a complete BPA degradation, we did not use the strong T7 promoter for expressing these BioBricks but a medium strong constitutive promoter (<partinfo>J23110</partinfo>). With this promoter upstream of a polycistronic bisdAB gene we were able to completely degrade 120 mg L-1 BPA in about 30 - 33 h. By fusing <partinfo>K123000</partinfo> and <partinfo>K123001</partinfo> together (using Freiburg BioBrick assembly standard) we could improve the BPA degradation of E. coli even further, so 120 mg L-1 BPA can be degraded in 21 - 24 h. This data is shown in the following figure:
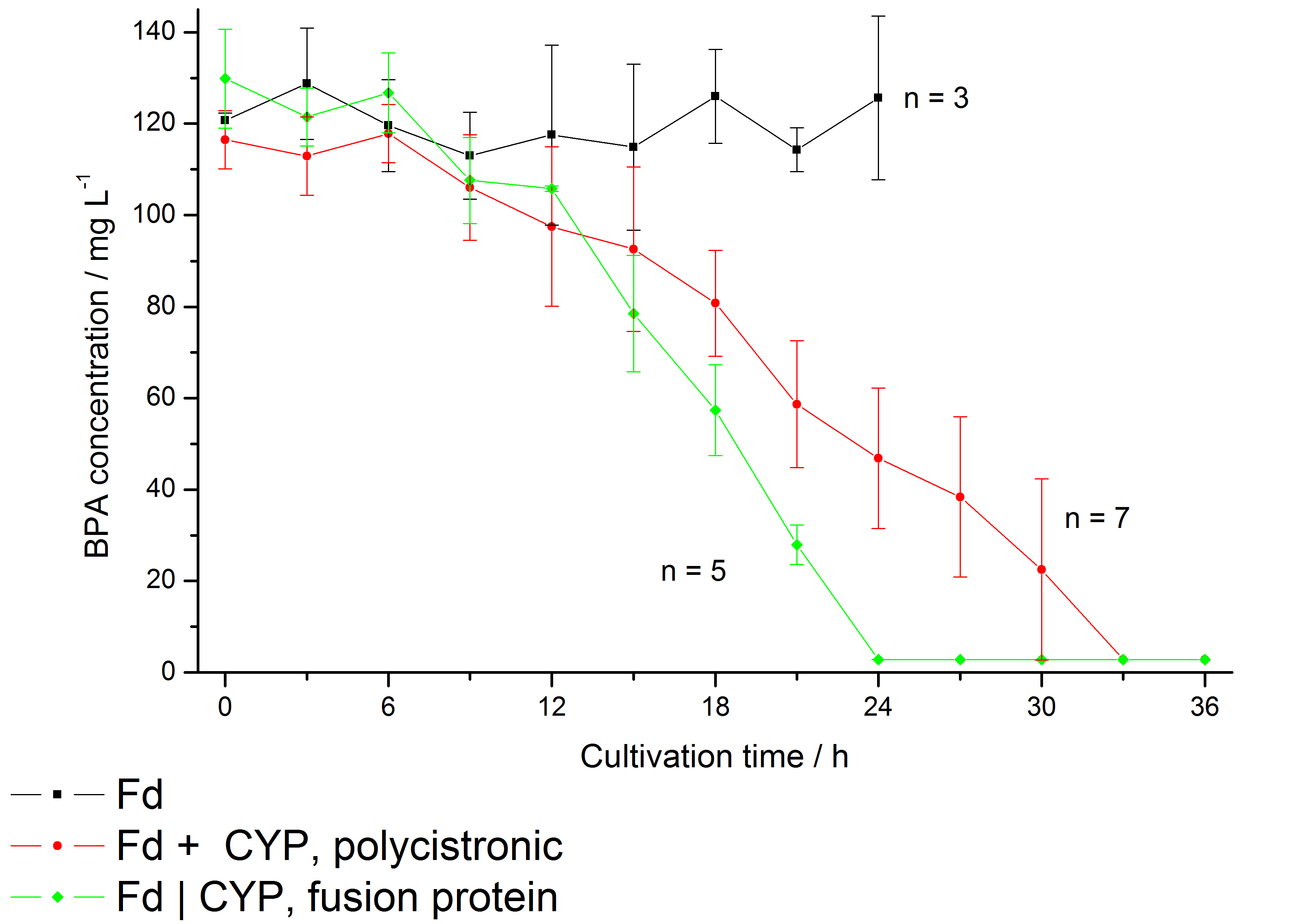
We also carried out these cultivations at different temperatures and BPA concentrations, but the chosen conditions (30 °C and 120 mg L-1 BPA) seem to be the best. Higher BPA concentrations have an effect on the growth of E. coli and higher temperature leeds to a worse BPA degradation (probably due to misfolding of the enzymes). These data on the effect of the temperature on the BPA degradation is shown in fig. 6.
 "
"




