Team:Bielefeld-Germany/Data Page
From 2011.igem.org
(Difference between revisions)
| Line 2: | Line 2: | ||
<html><img src="https://static.igem.org/mediawiki/2011/f/ff/Bielefeld-header-data-page.png"/><p></p></html> | <html><img src="https://static.igem.org/mediawiki/2011/f/ff/Bielefeld-header-data-page.png"/><p></p></html> | ||
| - | This page gives a basic overview about our cell-free Bisphenol A biosensor system and the BioBricks we have used. A more detailed description of the biosensor system can be found in our [[Team:Bielefeld-Germany/Project/Description | project description]] and in the [[Team:Bielefeld-Germany/Project/Background/BPA | Bisphenol A]], [[Team:Bielefeld-Germany/Project/Background/S-Layer | S- | + | This page gives a basic overview about our cell-free Bisphenol A biosensor system and the BioBricks we have used. A more detailed description of the biosensor system can be found in our [[Team:Bielefeld-Germany/Project/Description | project description]] and in the [[Team:Bielefeld-Germany/Project/Background/BPA | Bisphenol A]], [[Team:Bielefeld-Germany/Project/Background/S-Layer | S-layer]] and [[Team:Bielefeld-Germany/Project/Background/NAD | NAD<sup>+</sup> detection]] background subsections. |
| - | ==How our | + | ==How our system works== |
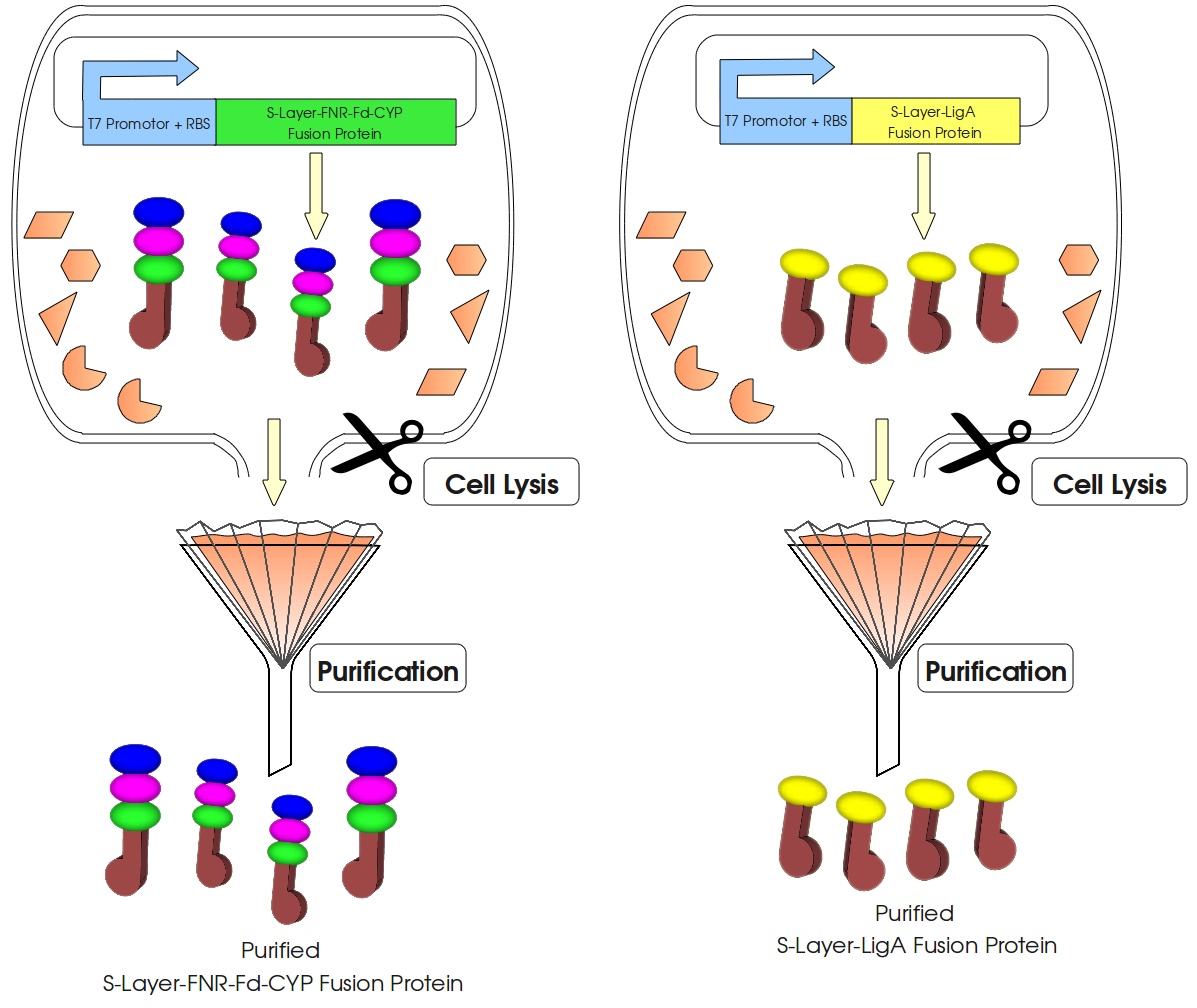
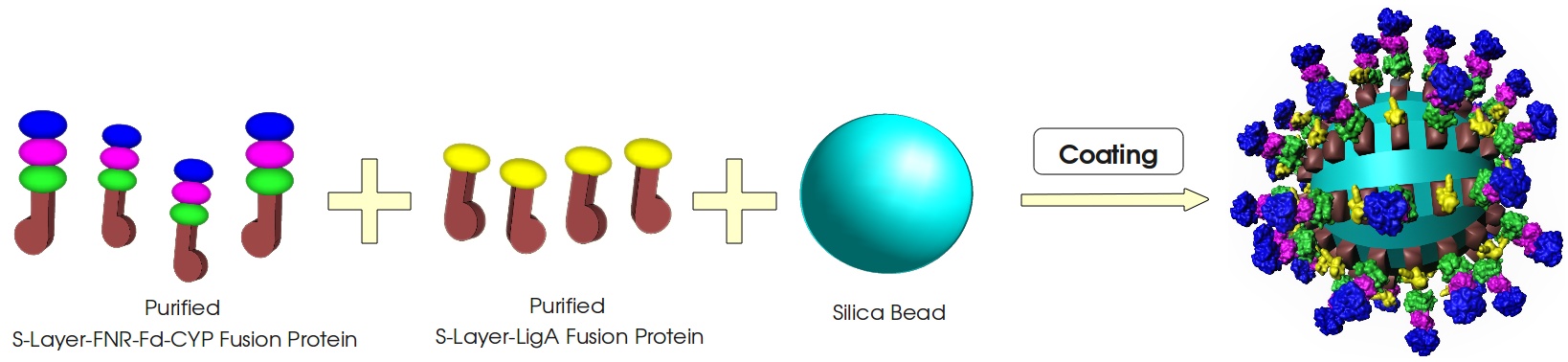
[[Image:Bielefeld_2011_S-Layer-Produktion_lysis-purification_v1.jpg|center|700px|thumb|'''Figure 1: Production of the S-Layer fusion proteins in ''E. coli''.''' To build a cellfree Bisphenol A biosensor the S-Layer fusion proteins have to be extracted from the cells and purified (simplified schema).]] | [[Image:Bielefeld_2011_S-Layer-Produktion_lysis-purification_v1.jpg|center|700px|thumb|'''Figure 1: Production of the S-Layer fusion proteins in ''E. coli''.''' To build a cellfree Bisphenol A biosensor the S-Layer fusion proteins have to be extracted from the cells and purified (simplified schema).]] | ||
| Line 14: | Line 14: | ||
| - | ==Data | + | ==Data for our favorite new parts== |
# [http://partsregistry.org/Part:BBa_K525305 Main Page] - '''Fusion Protein of S-Layer SgsE and mCitrine''': This fluorescent S-layer fusion protein is used to characterize purification methods and to demonstrate the S-layer's ability to self-assemble on surfaces. | # [http://partsregistry.org/Part:BBa_K525305 Main Page] - '''Fusion Protein of S-Layer SgsE and mCitrine''': This fluorescent S-layer fusion protein is used to characterize purification methods and to demonstrate the S-layer's ability to self-assemble on surfaces. | ||
# [http://partsregistry.org/Part:BBa_K525515 Main Page] - '''Fusion Protein of BisdA and BisdB''': This fusion protein improves the bisphenol A degradation in ''E. coli'' compared to the so far in the partsregistry existing BPA degrading BioBricks. | # [http://partsregistry.org/Part:BBa_K525515 Main Page] - '''Fusion Protein of BisdA and BisdB''': This fusion protein improves the bisphenol A degradation in ''E. coli'' compared to the so far in the partsregistry existing BPA degrading BioBricks. | ||
| Line 21: | Line 21: | ||
| - | ==Data | + | ==Data for pre-existing parts== |
# [http://partsregistry.org/Part:BBa_K123000:Experience Experience] - '''BisdA degrades Bisphenol A when used with BisdB, BBa_K123000''' (University of Alberta, iGEM 2008): Complete degradation of 120 mg L<sup>-1</sup> Bisphenol A with polycistronic ''bisdAB'' gene in 30-33 h. Even faster (21-24 h) when using a fusion protein of BisdA and BisdB. | # [http://partsregistry.org/Part:BBa_K123000:Experience Experience] - '''BisdA degrades Bisphenol A when used with BisdB, BBa_K123000''' (University of Alberta, iGEM 2008): Complete degradation of 120 mg L<sup>-1</sup> Bisphenol A with polycistronic ''bisdAB'' gene in 30-33 h. Even faster (21-24 h) when using a fusion protein of BisdA and BisdB. | ||
# [http://partsregistry.org/Part:BBa_K123001:Experience Experience] - '''BisdB degrades Bisphenol A when used with BisdA, BBa_K123001''' (University of Alberta, iGEM 2008): Complete degradation of 120 mg L<sup>-1</sup> Bisphenol A with polycistronic ''bisdAB'' gene in 30-33 h. Even faster (21-24 h) when using a fusion protein of BisdA and BisdB. | # [http://partsregistry.org/Part:BBa_K123001:Experience Experience] - '''BisdB degrades Bisphenol A when used with BisdA, BBa_K123001''' (University of Alberta, iGEM 2008): Complete degradation of 120 mg L<sup>-1</sup> Bisphenol A with polycistronic ''bisdAB'' gene in 30-33 h. Even faster (21-24 h) when using a fusion protein of BisdA and BisdB. | ||
| Line 27: | Line 27: | ||
| - | ==We | + | ==We have also characterized the following parts== |
# [http://partsregistry.org/Part:BBa_K525405 Main Page] - '''Fusion Protein of S-Layer SbpA and mCitrine''': This fluorescent S-layer fusion protein is used to characterize purification methods and to demonstrate the S-layer's ability to self-assemble on surfaces. | # [http://partsregistry.org/Part:BBa_K525405 Main Page] - '''Fusion Protein of S-Layer SbpA and mCitrine''': This fluorescent S-layer fusion protein is used to characterize purification methods and to demonstrate the S-layer's ability to self-assemble on surfaces. | ||
# [http://partsregistry.org/Part:BBa_K525512 Main Page] - '''Polycistronic expression of BisdA and BisdB''': This is the version of BPA degrading BioBricks found in the partsregistry - comparison to our fusion protein <partinfo>K525515</partinfo>. | # [http://partsregistry.org/Part:BBa_K525512 Main Page] - '''Polycistronic expression of BisdA and BisdB''': This is the version of BPA degrading BioBricks found in the partsregistry - comparison to our fusion protein <partinfo>K525515</partinfo>. | ||
# [http://partsregistry.org/Part:BBa_K525517 Main Page] - '''Fusion Protein of BisdA and BisdB (expressed)''': This fusion protein improves the bisphenol A degradation in ''E. coli'' compared to the so far in the partsregistry existing BPA degrading BioBricks. | # [http://partsregistry.org/Part:BBa_K525517 Main Page] - '''Fusion Protein of BisdA and BisdB (expressed)''': This fusion protein improves the bisphenol A degradation in ''E. coli'' compared to the so far in the partsregistry existing BPA degrading BioBricks. | ||
# [http://partsregistry.org/Part:BBa_K525234 Main Page] - '''Fusion protein of S-layer CspB and mRFP''': This fluorescent S-layer fusion protein is used to characterize the intracellular localisation as well as purification methods for CspB S-layers. | # [http://partsregistry.org/Part:BBa_K525234 Main Page] - '''Fusion protein of S-layer CspB and mRFP''': This fluorescent S-layer fusion protein is used to characterize the intracellular localisation as well as purification methods for CspB S-layers. | ||
Revision as of 11:32, 21 September 2011


This page gives a basic overview about our cell-free Bisphenol A biosensor system and the BioBricks we have used. A more detailed description of the biosensor system can be found in our project description and in the Bisphenol A, S-layer and NAD+ detection background subsections.
Contents |
How our system works
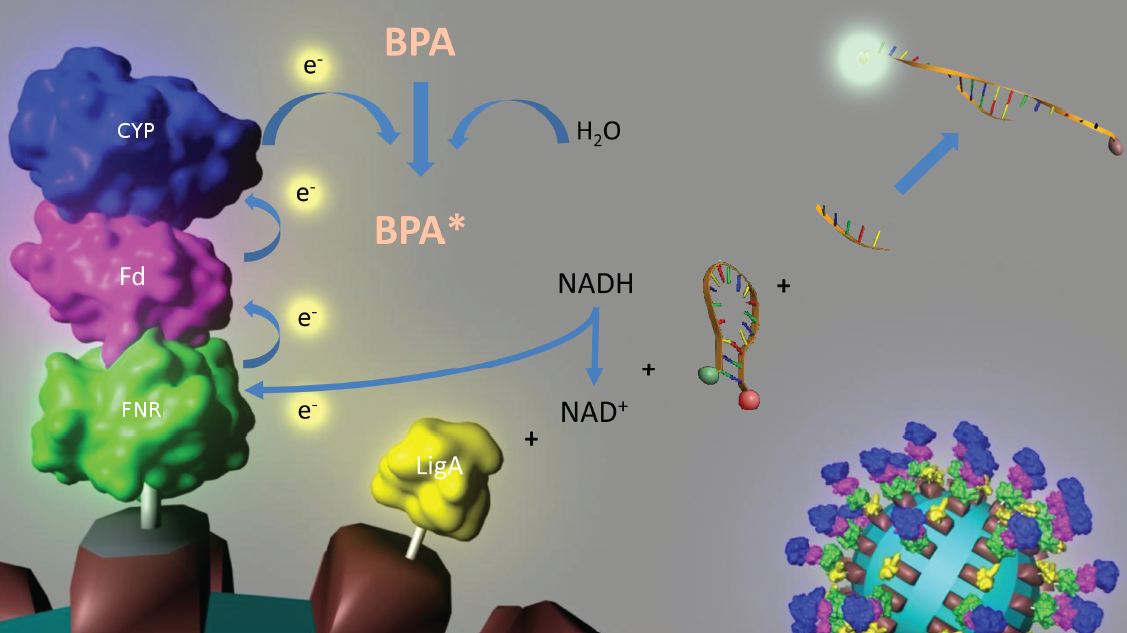
Figure 3: Visualization of our cell-free Bisphenol A biosensor system with all essential components. Bisphenol A (BPA) is reduced by the electrons from NADH transferred by the ferredoxin-NADP+ oxidoreductase (FNR, <partinfo>BBa_K525499</partinfo>), ferredoxin (Fd, <partinfo>BBa_K123000</partinfo>) and cytochrome P450 (CYP, <partinfo>BBa_K123001</partinfo>) which are fused to a S-Layer protein. The molecular beacon (hairpin structure) binds two short DNA oligos. The NAD+-dependent ligase (LigA), which is also fused to a S-Layer protein, ligates the two oligos so that the hairpin structure opens up and the fluorophore is able to emit light after extinction.
Data for our favorite new parts
- Main Page - Fusion Protein of S-Layer SgsE and mCitrine: This fluorescent S-layer fusion protein is used to characterize purification methods and to demonstrate the S-layer's ability to self-assemble on surfaces.
- Main Page - Fusion Protein of BisdA and BisdB: This fusion protein improves the bisphenol A degradation in E. coli compared to the so far in the partsregistry existing BPA degrading BioBricks.
- Main Page - NAD+-dependent DNA ligase from E. coli (LigA) : This enzyme enables determination of NAD+ even in very low concentrations by coupling it with a molecular beacon based bioassay.
Data for pre-existing parts
- Experience - BisdA degrades Bisphenol A when used with BisdB, BBa_K123000 (University of Alberta, iGEM 2008): Complete degradation of 120 mg L-1 Bisphenol A with polycistronic bisdAB gene in 30-33 h. Even faster (21-24 h) when using a fusion protein of BisdA and BisdB.
- Experience - BisdB degrades Bisphenol A when used with BisdA, BBa_K123001 (University of Alberta, iGEM 2008): Complete degradation of 120 mg L-1 Bisphenol A with polycistronic bisdAB gene in 30-33 h. Even faster (21-24 h) when using a fusion protein of BisdA and BisdB.
We have also characterized the following parts
- Main Page - Fusion Protein of S-Layer SbpA and mCitrine: This fluorescent S-layer fusion protein is used to characterize purification methods and to demonstrate the S-layer's ability to self-assemble on surfaces.
- Main Page - Polycistronic expression of BisdA and BisdB: This is the version of BPA degrading BioBricks found in the partsregistry - comparison to our fusion protein <partinfo>K525515</partinfo>.
- Main Page - Fusion Protein of BisdA and BisdB (expressed): This fusion protein improves the bisphenol A degradation in E. coli compared to the so far in the partsregistry existing BPA degrading BioBricks.
- Main Page - Fusion protein of S-layer CspB and mRFP: This fluorescent S-layer fusion protein is used to characterize the intracellular localisation as well as purification methods for CspB S-layers.
 "
"