Team:Bielefeld-Germany
From 2011.igem.org
| Line 9: | Line 9: | ||
</div> | </div> | ||
<div class="ws_bullets"><div> | <div class="ws_bullets"><div> | ||
| - | <a href="#wows0" title="group_foto4"><img src=" | + | <a href="#wows0" title="group_foto4"><img src="http://2011.igem-bielefeld.de/includes/wiki/tooltips/group_foto4.jpg" alt="group_foto4"/>1</a> |
| - | <a href="#wows1" title="Modell-final-final"><img src=" | + | <a href="#wows1" title="Modell-final-final"><img src="http://2011.igem-bielefeld.de/includes/wiki/tooltips/modellfinalfinal.jpg" alt="Modell-final-final"/>2</a> |
| - | <a href="#wows2" title="logo_auf_weiss"><img src=" | + | <a href="#wows2" title="logo_auf_weiss"><img src="http://2011.igem-bielefeld.de/includes/wiki/tooltips/logo_auf_weiss.jpg" alt="logo_auf_weiss"/>3</a> |
</div></div> | </div></div> | ||
<a style="display:none" href="http://wowslider.com">jQuery Slider Xml by WOWSlider.com v1.7</a> | <a style="display:none" href="http://wowslider.com">jQuery Slider Xml by WOWSlider.com v1.7</a> | ||
Revision as of 17:19, 11 August 2011

We care for biosecurity - check out our safety page.
Contents |
Project description
The development of sensitive and selective biosensors is an important topic in synthetic biology. Biosensors can be applied in a wide range - from the detection of environmental toxics up to clinical diagnostics. Because cells have to sense their surroundings, there are a lot of natural systems that are similar to a biosensor. Prejudicial cellular biosensors often show negative side effects that complicate any practical application. Common problems are the limited use outside a gene laboratory due to the use of genetically engineered cells, the low durability because of the usage of living cells and the appearance of undesired signals induced by endogenous metabolic pathways.
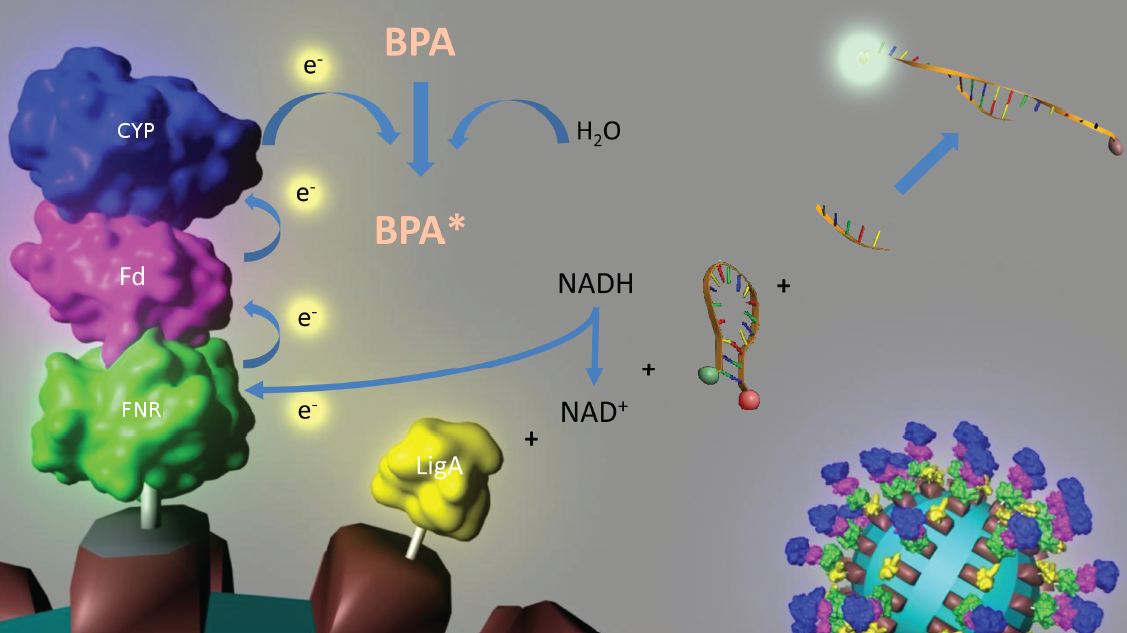
To solve these problems, the iGEM-Team Bielefeld 2011 aims at developing a cell-free Bisphenol A (BPA) biosensor based on a coupled enzyme reaction fused to S-layer proteins for everyday use. Bisphenol A is a supposedly harmful substance which is used in the production of polycarbonate. To detect BPA it is degraded by a fusion protein under formation of NAD+ which is detected by an NAD+ dependent enzymatic reaction with a molecular beacon. Both enzymes are fused to S-layer proteins which build up well-defined nanosurfaces and are attached to the surface of beads. By providing these nanobiotechnological building blocks the system is expandable to other applications.
An overview of our project is shown in the figure below. The background and the state of the art of each subproject is described below this figure. To have a quick insight of what is happining in our lab take a look in our Labjournal.
S-layer
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S layer geometry) it is possible to realize various practical applications. The usability of such well defined nano-lattice structures is far-reaching from ultrafiltration membranes to the development of immobilized biosensors.
Especially for the production of cell-free biosensors, functional fusion proteins are of great importance. Sleytr et al. fused fluorescent proteins with an S-layer glycoprotein from Geobacillus stearothermophilus. They demonstrated that the properties of the fusion protein were similar to the native fluorescent protein. The intensity of the fluorescence, the lifetime and the adsorption spectra showed comparable behavior at different pH-values. Enzymes fused to immobilized S-layers showed a significantly longer durability.
The iGEM-Team Bielefeld aims at the assembly, production and immobilization of S-layer fusion proteins for the detection of BPA by a coupled enzymatic reaction. S-layers from five different organisms are employed. The provision of various S-layers with different geometries offers the possibility for the scientific community to create functional nanobiotechnological surfaces with simple and standardized methods, quasi do it yourself nanobiotechnology. First, different fusion proteins with fluorescent proteins and a luciferase are created. The functionality and efficiency of the immobilization to various materials such as silicon dioxide or cellulose is then characterized by measuring the fluorescence and luminescence, respectively.
Bisphenol A degradation
In 2005, Sasaki et al. isolated a soil bacterium from the Sphingomonas genus which is able to degrade the environmental poison Bisphenol A (BPA) with a unique rate and efficiency compared to other BPA degrading organisms. This strain was called Sphingomonas bisphenolicum AO1 and is able to completely decompose 120 mg BPA L-1 in about 6 hours. Three genes which are responsible for the first step of this effective BPA degradation by S. bisphenolicum AO1 were identified: a cytochrom P450 (bisdB), a ferredoxin (bisdA) and a ferredoxin-NAD+ oxidoreductase (Red). The bisdAB genes from S. bisphenolicum AO1 were isolated, transformed into and expressed in E. coli and enabled this bacterium to degrade BPA, too. In addition, the BisdAB proteins from S. bisphenolicum AO1 were able to degrade BPA in a cell free system in which spinach reductase was added. So we assume that the BisdAB proteins also work in a cell free system together with the ferredoxin-NAD(P)+ oxidoreductase from E. coli. The suggested reaction mechanism of the first BPA degradation step is shown in the project overview image above.
In 2008, the iGEM team from the University of Alberta submitted the codon usage optimized bisdAB genes from S. bisphenolicum AO1 to the registry of standard biological parts in the so called [http://partsregistry.org/Assembly_standard_25 Freiburg BioBrick assembly standard] (<partinfo>K123000</partinfo> and <partinfo>K123001</partinfo>). Via this assembly standard it is very easy to build fusion proteins. These already existing protein domains will be fused together with the NAD(P)+ oxidoreductase gene from E. coli to the fusion protein Red:Fdbisd:P450bisd which subsequently will be fused to an S-layer gene. We have already shown that the Fdbisd:P450bisd fusion protein is degrading BPA more effective in E. coli than the polycistronic bisdAB gene (data not shown).
NAD+ detection
Our selected NAD+ detection method displays a molecular beacon based approach. These have been initially described in 1996 as nucleic acid probes that fluoresce upon hybridization. For this effect the ends of a single-stranded DNA molecule are labeled with a fluorophore as well as with an appropriate quencher. Both are in close proximity to each other due to a formed stem-loop, so that the detection of any fluorescence signal is prevented.
The molecular beacon’s closed state can be applied to a bioassay detecting NAD+ even in very low concentrations (LOD 0,3 nM). Using two complementary targets hybridizing side-by-side with the hairpin enables NAD+-dependent DNA ligation by E. coli DNA ligase. Only after closing the gap between both hybridized targets the stem melts and the secondary structure gets broken down to a linearized probe-target hybrid. The immediate consequence is a disruption of the close proximity of the fluorophore and the quencher, so that an excitation with light is converted into a visible fluorescence signal. Hence, NAD+ concentration determines DNA ligase activity, which is responsible for the formation of the molecular beacon’s open state and therefore directly correlates with the emerging fluorescence signal. Additionally, the highly selective bioassay has a low limit of detection compared to other methods.
Because of the signal’s stability and the suitability for daily use it can be coupled to NADH-dependent BPA degradation in the context of biosensing.
6th Cebitec Symposium
European iGEM teams meet in Bielefeld, Germany A review on the 6th CeBiTec Symposium Genome-Based Microbiology: From -omics Research to Systems and Synthetic Biology
The annual CeBiTec Symposium, first held in 2006, deals with prospective topics in the broad spectrum of biotechnological research. Each year international scientists come together in Bielefeld setting the focus on current innovations, approaches and methodologies. The issues so far included Solar Bio-Fuels (2008), bioIMAGING (2009) and New Frontiers in Microbial Genome Research (2010). The 6th CeBiTec Symposium was held under the topic Genome-Based Microbiology: From -omics Research to Systems and Synthetic Biology took place between the 18th and 20th of July, 2011. As this year’s topic included Synthetic Biology, this offered the opportunity for the iGEM team Bielefeld to add an iGEM session to the symposium’s agenda. The students accommodated five teams from all over Europe to present and discuss their past and future projects in front of the international scientific community.
Starting with one of the last year’s finalist TU Delft’s 2010 iGEM project about the conversion of different hydrocarbon sources in aqueous environments, all attending teams presented their results and strategies. The team from the University of Southern Denmark, Odense, presented their 2010’s project Flow E. The aim was to develop a biological system, which is able to generate flow in a fluid-filled tube by inducing the flagella movement with light by the use of a photosensor. This year’s iGEM Team from the University of Edinburgh aims to use and convert plant biomass, especially cellulose, to fermentable sugars like glucose. Therefore several cellulases are fused to a coat protein of the M13 bacteriophage. Ultimately the team’s goal is to create a biorefinery system to convert complex glucans into higher value products. The last year’s medicine track winner Freiburg presented their virus construction kit for therapy use. They managed to create different BioBricks and devices for individual virus construction. Last year’s grand prize winner Slovenia gave the audience a brief historical overview of all Slovenian iGEM projects so far. The session closed with the talk of the Team Bielefeld dealing with their topics from 2010 – a modulated two component sensor system for detection of capsaicin – and a preview for 2011 – nanobiotechnolgical S-layer based Biosensor for bisphenol A.
Additionally, the students used the opportunity to share and exchange their iGEM experience and their ideas of Synthetic Biology at the symposium and at the social events provided by the iGEM Team Bielefeld. The symposium’s iGEM session was a success and hence indices that the iGEM idea reached the scientific community.
Some expressions from the CeBiTec Symposium:
 "
"

 Jojojo das ist eine Test-description
Jojojo das ist eine Test-description Auch hier ist eine Test-description vorhanden
Auch hier ist eine Test-description vorhanden Das selbe wie imme, ne...
Das selbe wie imme, ne... 1
1 2
2 3
3