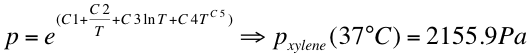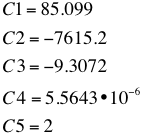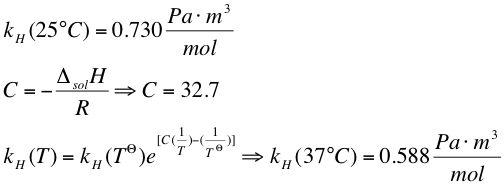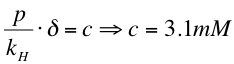Team:ETH Zurich/xylene
From 2011.igem.org
(Difference between revisions)
(Created page with "{{:Team:ETH Zurich/Templates/HeaderNew}} {{:Team:ETH Zurich/Templates/SectionStart}} == How we calculated the concentration of m-xylene in the medium... == 1. We assumed that ...") |
|||
| Line 7: | Line 7: | ||
1. We assumed that 5 ml xylene is enough to saturate the gas phase in the Erlenmeyer flask. | 1. We assumed that 5 ml xylene is enough to saturate the gas phase in the Erlenmeyer flask. | ||
:The vapor pressure was calculated at 37 °C: | :The vapor pressure was calculated at 37 °C: | ||
| - | [[File:Dampfdruck.png|600px| | + | [[File:Dampfdruck.png|600px|left|thumb]] |
:with the following C1-C5 values: | :with the following C1-C5 values: | ||
:[[File:ETH Dampfdruck c.png|600px|left|thumb]] | :[[File:ETH Dampfdruck c.png|600px|left|thumb]] | ||
| Line 16: | Line 16: | ||
3. By Henry´s law we calculated the concentration of xylene in the medium | 3. By Henry´s law we calculated the concentration of xylene in the medium | ||
:[[File:Henry law.png|600px|left|thumb]] | :[[File:Henry law.png|600px|left|thumb]] | ||
| + | <br clear="all" /> | ||
4. For the oil/xylene mixtures we used Raoult's law | 4. For the oil/xylene mixtures we used Raoult's law | ||
[[File:Raoult's law.png|600px|left|thumb]] | [[File:Raoult's law.png|600px|left|thumb]] | ||
{{:Team:ETH Zurich/Templates/SectionEnd}} | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
Revision as of 07:53, 27 October 2011
How we calculated the concentration of m-xylene in the medium...
1. We assumed that 5 ml xylene is enough to saturate the gas phase in the Erlenmeyer flask.
- The vapor pressure was calculated at 37 °C:
- with the following C1-C5 values:
2. The Henry´s constant at 37 °C can be calculated by the Van´t Hoff equation from the literature value at 25°C
3. By Henry´s law we calculated the concentration of xylene in the medium
4. For the oil/xylene mixtures we used Raoult's law
 "
"