Team:ETH Zurich/Biology/Journal
From 2011.igem.org

Lab Journal
Here you can follow the progress of our project as it is coming together.
Week 3: 4.7-10.7
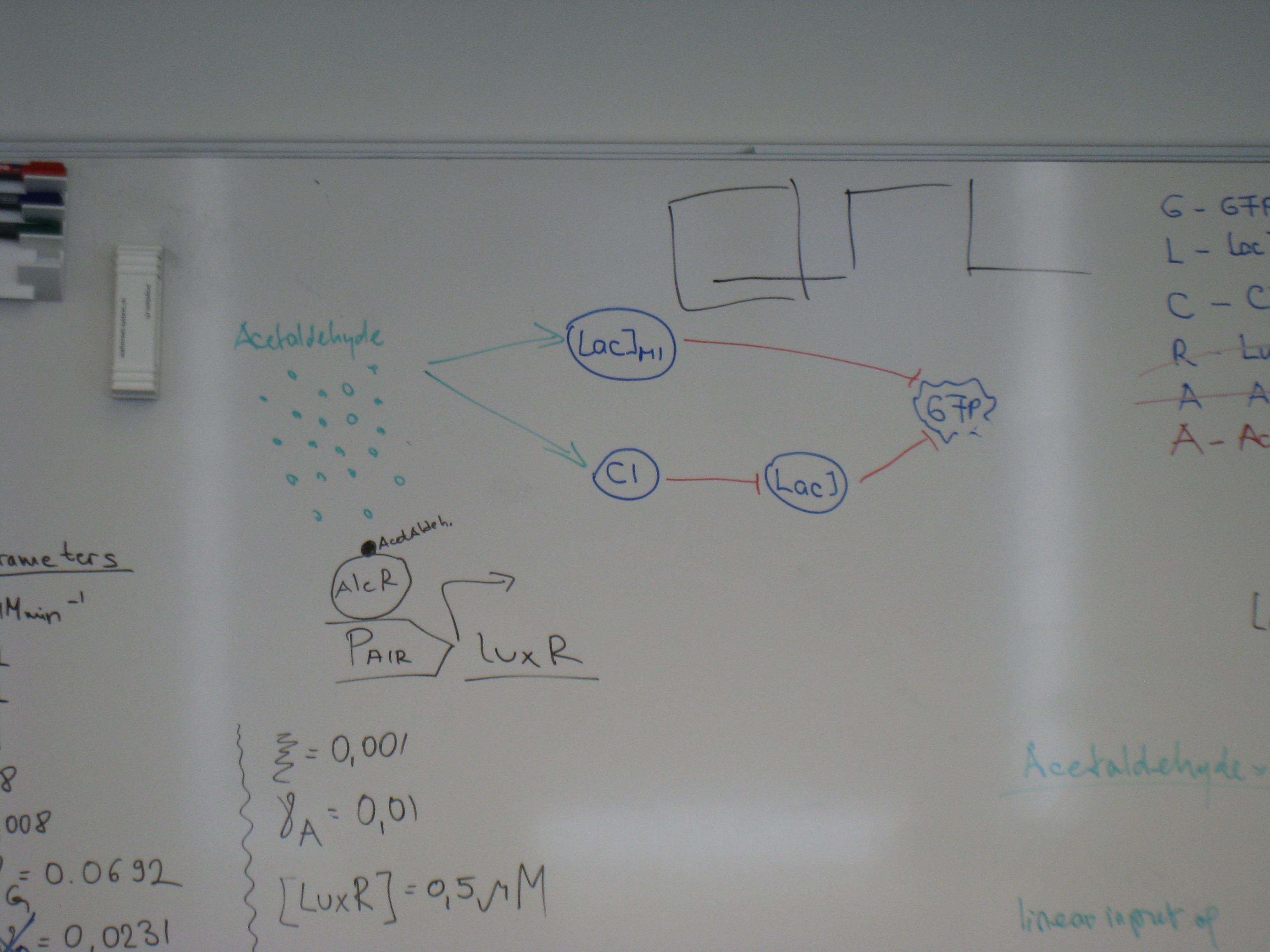
- Working on the design of the system
- Design of several Operons for AlcR and several PCR primers
Week 4: 11.7-17.7
- Ordering of the LacIM1 and codon-optimized AlcR gene
- Making of competent DH5-α cells
- Cloning of the AlcR-testsystem
Week 5: 18.7-24.7
- First test of the AlcR-system in 96-well plates
Week 6: 25.7-31.7
- Transformation of the parts from the iGEM plates
Week 7: 1.8-7.8
- Growth test of DH5-α with AlcR-system in flasks
- Ligation and Transformation of
- PR and luxI
- PR and lacI
- Plac and GFPLVA
- Plux and mCherry
- Ptet and CI -had to be redone because of point mutation in the primer (week 9)
- Pconst and luxR
- First meeting with Dr. Oliver Frey (Bio engineering laboratory, Prof. Andreas Hierlemann, D-BSSE ETHZ) to exchange ideas about microfluidic design
Week 8: 8.8-14.8
- Synthesized LacIM1 Gen arrived
- Ligation and Transformation of
- Plac-GFPLVA and Terminator
- Plux-mCherry and Terminator
- Pconst-luxR and Terminator
- Plac-GFPLVA-Terminator and Plux-mCherry-Terminator on one plasmid
- Ptet and LacIM1
- Ptet-LacIM1 and Terminator
- Improving of the testsystem
- Exchange of some rare codons in AlcR by PCR
- His-tagging of AlcR for detection in Western blot
- include sfGFP
- Transformation of PR
- Creation of competent JM101 cells
- Check for transformation efficiency
Week 9: 15.8-21.8
- Growth and repression test of AlcR-system with M9-medium
- Ligation and Transformation of
- PR and lacI
- PR and luxI
- Ptet and CI
- PR-lacI and terminator
- PR-luxI and terminator
Week 10: 22.8-28.8
- Transformation and Ligation
- Ptet-CI and Terminator
- Preparation of chemically competent DH5-α and JM101 cells
Week 11: 29.8-4.9
- Transformation and Ligation
- Ptet-CI and Terminator
- Diffusion test through tube system filled with agarose and cells
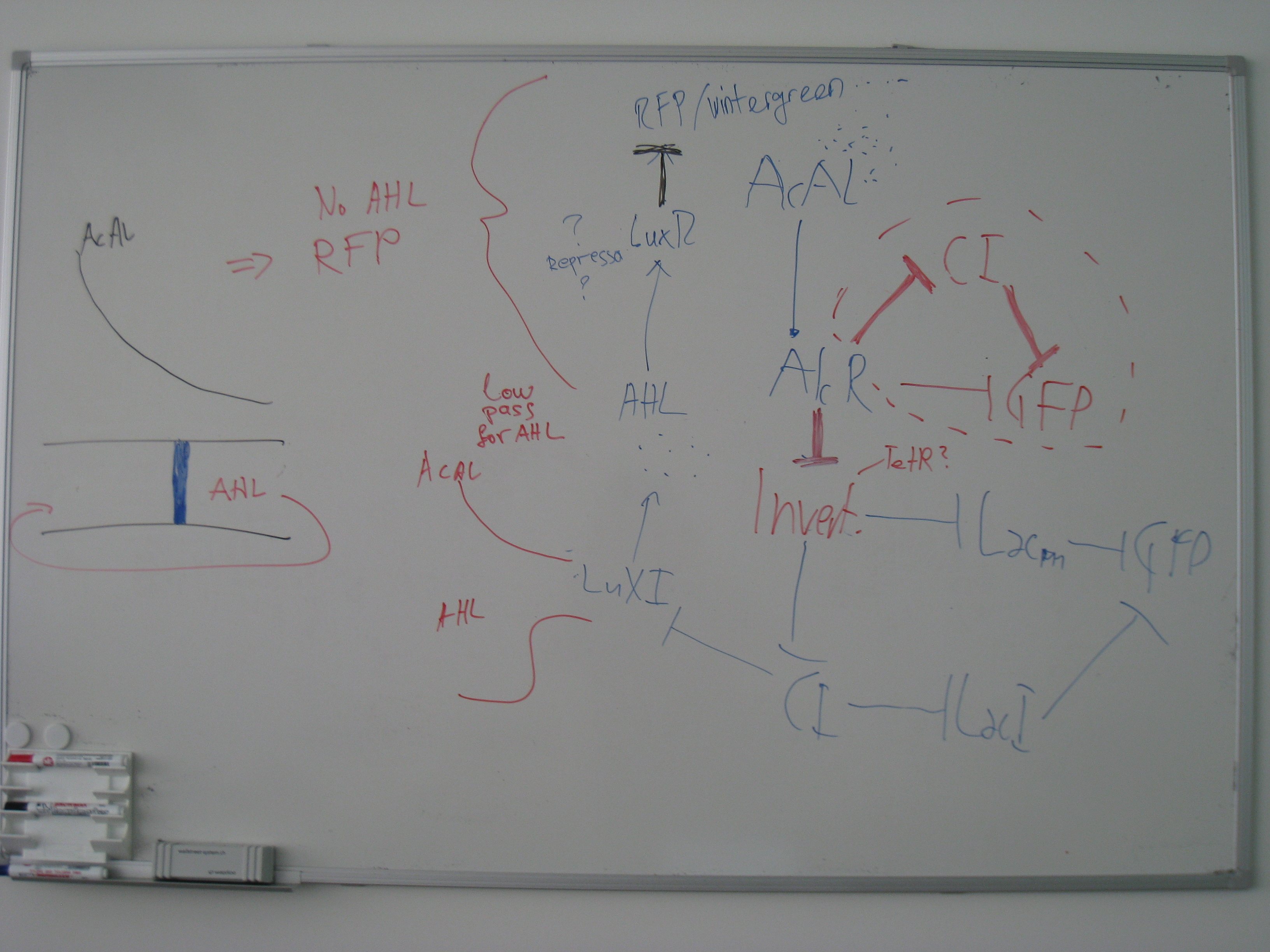
- Test of the improved AlcR system
Week 12: 5.9.-11.9
- Design of a system to characterize our new biobrick LacIM1
- Transformation and Ligation
- PTet and LacI
- PLac and GFP assam
- Transformation and Ligation
- Design of an additional construct to test the band pass filter
- Transformation and Ligation
- Pconst and TetRLVA
- Transformation and Ligation
- Growth-test of E. coli in M9 with reduced Ampicillin concentration
- AlcR test with reduced Ampicillin concentration
- Testdigest of λP-luxI-Terminator
- Alternative Sensor system: Xylene system
- Transformation and Ligation
- Pconst and XylR
- PXyl and CI
- PXyl and LacI M1
- Transformation and Ligation
Week 13: 12.9.-18.9
- PCR of the pBR322 Ori
- PCR of the gen cluster for the upper TOL pathway
- first test of cell printing
- Transformation and Ligation of
- Pconst-TetR- PTet-LacIM1 and PR-GFPassam
- Western Blot of AlcR
- Making of competent DH5-α cells
- Design and construction of Psb6A5
Week 14: 19.9.-25.9
- Characterization of LacIM1
- Cloning of all biobricks into pSB1C3
- PCR of LacI and CI
- wiki
Week 15: 26.9.-02.10
- design of Pu promoter
- test of Pu promoter
- test for indol degradation
- Poster
- Preparing the presentation
European Jamboree 30.9-2.10
- Sightseeing and having fun in Amsterdam
- Gold Medal
- Honorable Mention in category Best Model
- Jamboree
Week 16: 03.10.-09.10
- proof of concept with an arabinose inducable system
- cloning for the xylene system
- cloning of the lac-testsystem to compaire LacIM1
Week 17: 10.10.-16.10
- cloning of the alarm-test system
- cloning the final system with xylene
- cloning the arabinose system
- cloning the AlcR (codon optimized) system
- building a channel out of different materials
- Design a assay for testing the alarm system
- Sender receiver test on plates
Week 18: 17.10.-23.10
- Cloning
- inactivating of the kanamycin casette of BW27749 to get BW27783
- Make competent BW27783 and DH5-α cells
- Sender receiver test on plates with sterile filtrated supernatant
- Sender an receiver test in tube
- Dose response experiment for xylene
- Dose response experiment for acetaldehyde
Week 19: 24.10-30.10
- Cloning
- Microscope experiments of the channel with and without degradation
- Alarm test with AHL
- Dose response experiment for acetaldehyde
- Dose response experiment for arabinose
 "
"