Team:ETH Zurich/Process/Microfluidics
From 2011.igem.org
(→Final Design: Microfluidics channel with PDMS) |
(→Final Design: Microfluidics channel with PDMS) |
||
| Line 90: | Line 90: | ||
Finally, we decided to construct our channels out of PDMS (polydimethylsiloxane), with a technique called photolithography. Advantages of PDMS are that it is cheap, optically clear and permeable to several substances, including gases (air can quickly diffuse through) [[#Ref1|[1]]]. <br><br> | Finally, we decided to construct our channels out of PDMS (polydimethylsiloxane), with a technique called photolithography. Advantages of PDMS are that it is cheap, optically clear and permeable to several substances, including gases (air can quickly diffuse through) [[#Ref1|[1]]]. <br><br> | ||
| - | We constructed the channels ourselves, which was very fun and interesting process. Our final design and the | + | We constructed the channels ourselves, which was a very fun and interesting process. Our final design and the channel construction is explained in details [[Team:ETH_Zurich/Process/Validation|here]]. |
{{:Team:ETH Zurich/Templates/SectionEnd}} | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
{{:Team:ETH Zurich/Templates/SectionStart}} | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
Revision as of 19:37, 27 October 2011
Microfluidic Channel Design
For implementation of the SmoColi system, a channel is needed to establish a small molecule gradient (see Circuit Design). However, there were several different possible channel designs, and the final design evolved through an iterative series of design steps and design validations. The first designs were validated based on vast simulations, the final design furthermore by biological experiments in the lab (see Systems Validation).
Microfluidic channel with flow and recycling of the medium
We came up with two different possible microfluidic channel designs, both involving immobilized cells and a flow of medium containing inducer molecules through the channel. By having a flow and degradation, we could obtain a gradient of the inducer molecule. Because of the flow, our cells would also be constantly supplied with nutrients from the medium.
- Variant 1: Microfluidic channel with agarose-immobilized cells in cubic pockets
This channel design consists of two layers: The bottom one is a polydimethylsiloxane (PDMS) plate with regular cubic pockets. These pockets are filled with agarose-immobilized cells by letting the cell-agarose suspension flow over the pockets from the top by gravity. After washing the channel, cells would only be retained in the pockets.
The top layer is the actual PDMS channel, which is several pixels wide and several pixels high. Although this setup is rather complex, it has the advantage of having immobilized cells and thus being more robust, i.e. we could vary flow speeds or even put aerosol in the channel without the cells being washed out. Additionally, cell density can be varied very easily in this design as the cells can be diluted before being immobilized in agarose.
- Variant 2: Microfluidic channel with cells sitting in pockets inside the channel
This channel design only consists of one part: A PDMS channel that is fixed onto a glass carrier. The PDMS channel contains pockets which "trap" the cells given a constant flow from the direction of the "open end" of the pockets. This channel design does not have the advantages of the above one, i.e. it is not as robust and cell density cannot be reliably varied. However, manufacturing it is a standard process and thus it is easier.
Problems with these design variants:
A problem with both of these designs is that for the AHL-based RFP alarm to work, recycling of the flow back into the channel would be required. AHL-producing cells are only those "after" the GFP band, i.e. those at lower acetaldehyde concentration than the band concentration range. As long as the GFP band has not arrived to the end of the channel, we should make sure that there is AHL everywhere in the channel, so that it inhibits RFP production in every cell. Modeling showed that AHL can not simply diffuse "backwards" against the flow, but by having a recycling all the cells would be supplied with AHL and thus the alarm won't be activated before time.
However, since the tubing and pumps would have very high volumes compared to the channel's volume, the AHL signal would be diluted to the point where no detection is possible anymore. Also, several pumps would be required to accomplish this, further complicating the process design and making it more error-prone.
Improved microfluidic channel without flow
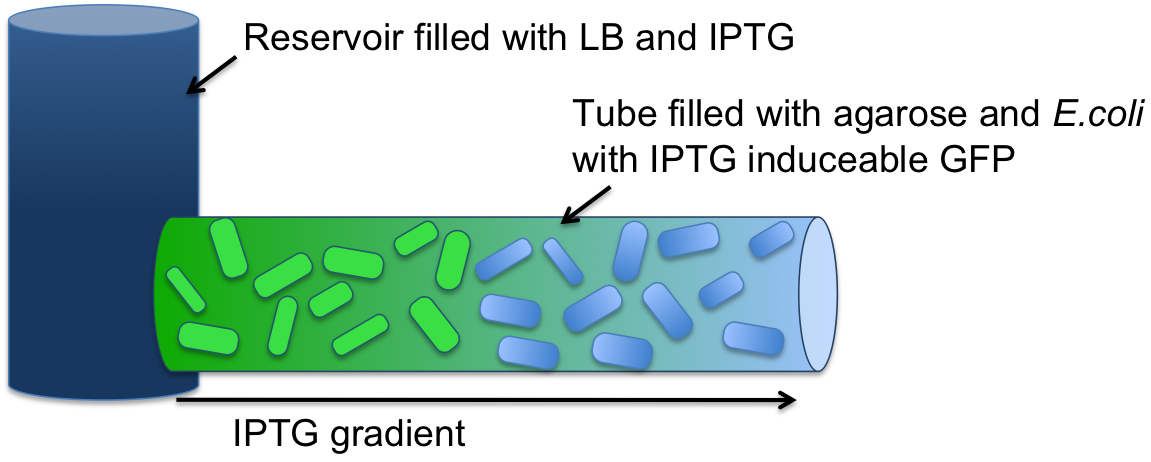
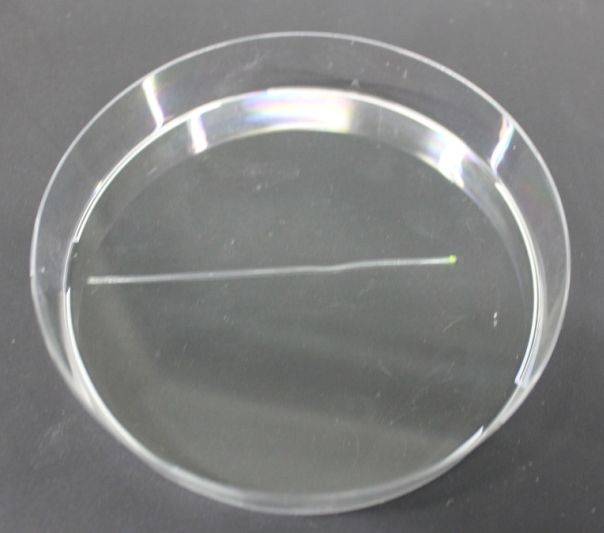
Modeling showed that diffusion and degradation of the inducer are enough to create a concentration gradient in the channel. The experimental validation of this hypothesis was first performed in a 2 mm diameter tube. For proof of concept, we engineered E. coli strain JM101 to express GFP upon IPTG induction. The cells were then immobilized in agarose and the suspension was added to the tube (see Figure 4). One end of the tube was connected to a reservoir (1.5 ml) containing 10 mM IPTG solution. After overnight incubation at 37 °C, an IPTG-inducible GFP gradient was observed (Figure 6 and 7). The experiment confirmed the modeling results. Our cells survived and we concluded that we do not need constant supply of nutrients.
Without a flow there is no need for a moving liquid and thus no need for any of the complex designs above, as one can simply fill the whole channel with cell-agarose solution. We can then wait until the cell-agarose solution solidifies to a gel and then connect one side of the channel to a reservoir with the toxic molecule, while sealing the other. Likewise, we do not need recycling because AHL can diffuse through the whole channel and does not have to diffuse against a flow. Moreover, having a tube instead of a microfluidic device would save us some time that we would need otherwise for the channel construction.
Modeling the system thus had a profound effect on the process design, leading to an extensive reduction of complexity and error-proneness. Additionally, the AHL recycling idea would not have worked in the initial design, and was "saved" by the new channel design we developed by modeling our system.
Fluorescence pictures of the tube showed a clear gradient of the fluorescence signal over approximately 5 cm of the tube. After 5cm, the signal strength dropped under the background noise.
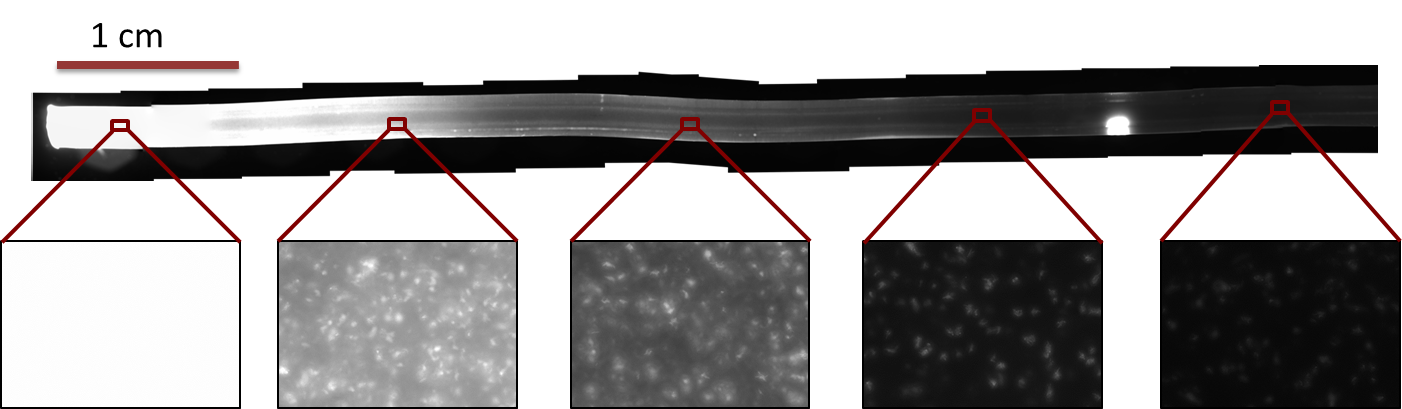
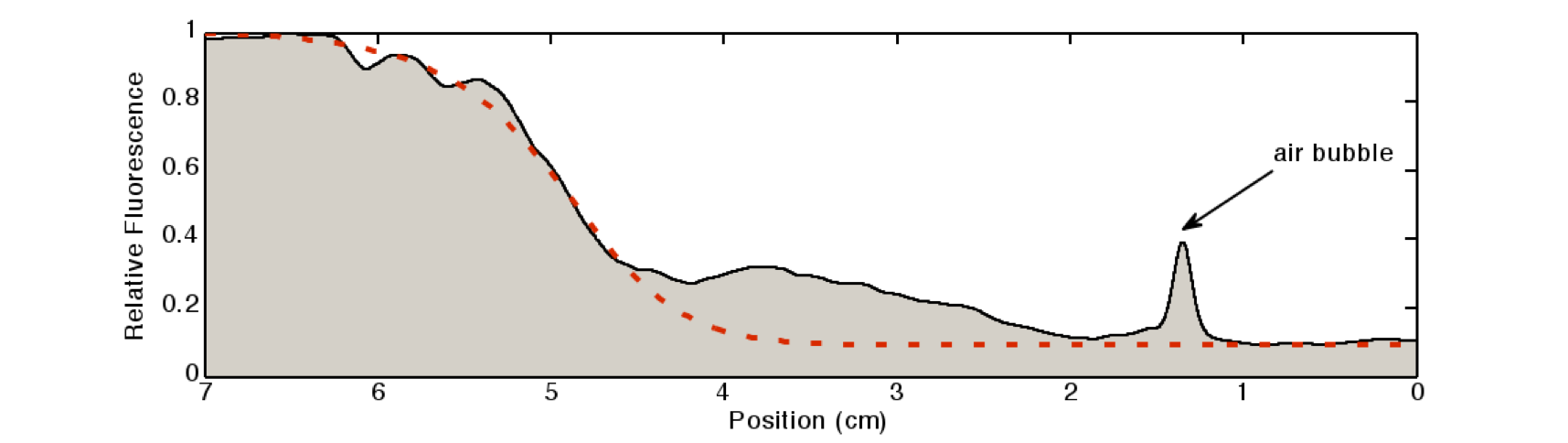
We quantified the fluorescence signal using a moving average of 80×80 pixel, which moved along the symmetry axis of the tube (see Figure 7), in red you can see the according reaction diffusion model.
The fluorescence distribution of this experiment has a similar shape as the distributions predicted by the model (see modeling section). The difference in the experimental results compared to the simulations can be explained mainly due to the different diffusing molecules: the simulations were obtained for acetaldehyde, whereas the experiments were carried out with IPTG. We expect the different values of the diffusion as well as of the degradation constants of the two molecules to be the main reason for the differences.
Problem with this design variant: No live imaging is possible, but only end point measurements, since the agarose with cells first has to be taken out of the tube and then analyzed under microscope.
Further improvements: Microfluidic Channels with Silicon glue
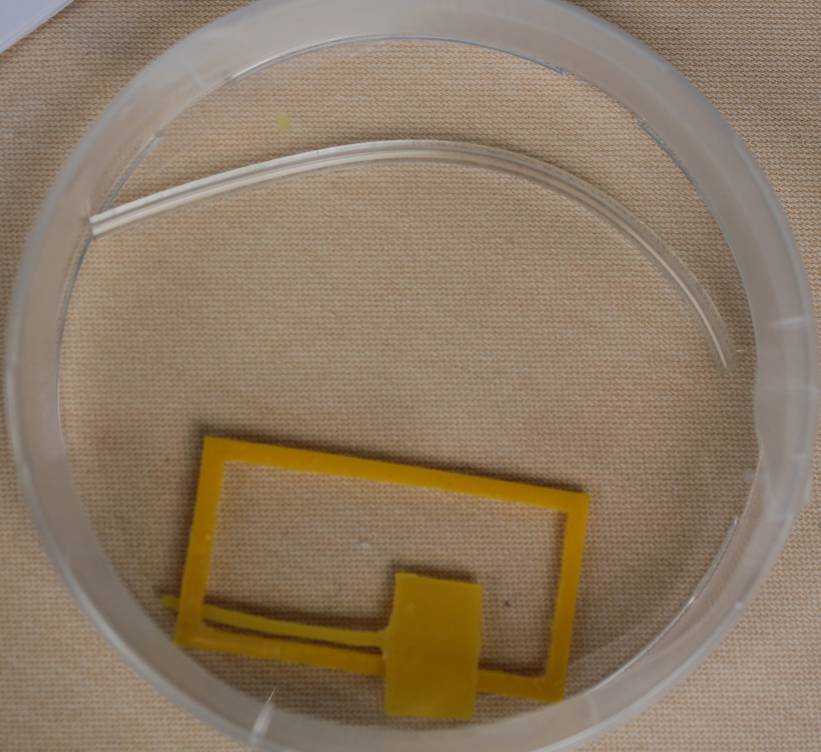
Since with the tube live imaging is not possible, we returned to microfluidics again and improved our channel design further. In order to image the channel at its top and bottom a glass slide is needed. We tried to build the channel by using a plastic mask and casting the boundaries with different materials. We used a two-component glue made out of silicon and molten parafilm. The glue turned out to be toxic for our cells. Silicon and molten parafilm in contrast seem to be adequate for our channel.
Problem with this design:
Toxicity.
At the same time we tried something more frequently used, Polydimethylsiloxane (PDMS) for constructing the channel, which later turned out to be our final design.
Final Design: Microfluidics channel with PDMS
Finally, we decided to construct our channels out of PDMS (polydimethylsiloxane), with a technique called photolithography. Advantages of PDMS are that it is cheap, optically clear and permeable to several substances, including gases (air can quickly diffuse through) [1].
We constructed the channels ourselves, which was a very fun and interesting process. Our final design and the channel construction is explained in details here.
References
[1] [http://www.elveflow.com/microfluidic/16-start-with-microfluidic http://www.elveflow.com/microfluidic/16-start-with-microfluidic]
 "
"