Team:Bielefeld-Germany/Results/NAD
From 2011.igem.org
(→NAD+ detection) |
|||
| Line 79: | Line 79: | ||
| - | [[Image:Bielefeld-Germany- | + | [[Image:Bielefeld-Germany-2011_NAD+_calibrationcurvepng.png|650px|thumb|centre| '''Figure 6: Initial velocity calibration curve.''' The initial velocity, calculated from the average fluorescence enhancement rate in 200 s after NAD<sup>+</sup> addition as shown in figure 2, is plotted versus the employed NAD<sup>+</sup> concentration. Data is fitted with linear regression for NAD<sup>+</sup> concentrations ranging from 0 to 200 nM (R²=0.991, n=4).]] |
Revision as of 16:33, 20 September 2011


Contents |
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression | ||
| Compatibility | E. coli KRX | |
| Promoter | PT7 | |
| Inductor | L-rhamnose | |
| Optimal temperature | 37 °C | |
| Purification | ||
| Number of amino acids | 678 | |
| Molecular weight | 74.59 kDa | |
| Theoretical pI | 5.59 | |
| Extinction coefficient at 280 nm measured in water [M-1 cm-1] | 37400 (assuming all pairs of Cys residues form cystines)
36900 (assuming all Cys residues are reduced) | |
| Tag | C-terminal 6xHis | |
| NAD+ detection | ||
| Minimal employed NAD+ concentration | 5 nM |
Purification of LigA
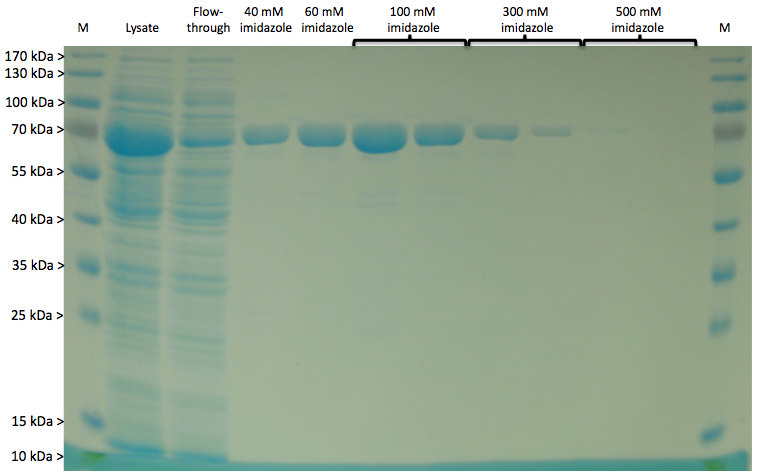
Expression of LigA in E. coli is an appropriate opportunity to produce the recombinant protein LigA in a large scale. The protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0,1 % rhamnose. Cell harvest was performed after 4 h further growth at 37 °C. After cell lysis the protein could be purified with the help of Ni-NTA columns utilizing the proteins C-terminal His-tag. As shown in figure 1 the majority of LigA was eluted with 100 mM imidazole. Usually the protein occurs in its adenylated form after extraction from E. coli. Depending on whether the protein should be in its deadenylated form for further applications a treatment with an excess of NMN for deadenylation is possible.
NAD+ detection
LigA can be applied to a molecular beacon based bioassay detecting NAD+ even in very low concentrations. For this two important requirements have to be fulfilled: the purified LigA has to be in its deadenylated form so that DNA ligation occurs only in presence of NAD+. Furthermore, molecular beacons have to be designed appropriately so that they stay in their closed state under the chosen conditions and after hybridization to a split target. Not before LigA ligates the split target in a NAD+-dependent manner the molecular beacon is supposed to form its open state resulting in an increase of fluorescence intensity.
The proof for a valid NAD+ detection system which has been used for characterization of LigA is shown in figures 2 and 3. The molecular beacon formed its open state after hybridization to a complementary target so that the close proximity of the fluorophor and the quencher broke up. After excitation with light a 45.52-fold increase in fluorescence intensity compared to the background signal (molecular beacon alone) could be determined. By contrast, this was not the case after using a complementary split target hybridzied to the molecular beacon because the signal-to-background ratio was just 3.36. This shows that the split target was not able to reach the molecular beacons open state, but the complete target did so. Thus, this approach is useful to measure LigA activity and therefore detect NAD+.
For the NAD+ bioassay the molecular beacon was preincubated with the split target at 37 °C. After the fluorescence intensity reached equilibrium purified and deadenylated LigA was added in a final concentration of 5 ng/µL followed by NAD+ addition (figure 4). First of all, there was only little increase in flourescence intensity after LigA addition indicating that the pretreatment with NMN for deadenylation was successful. In a separate experiment with LigA that was not pretreated with NMN a continous increase in fluorescence intensity was observed (data not shown). This leads to the suggestion that an excess of NMN supports deadenylation reaction of LigA which makes the enzyme suitable for NAD+ detection. After NAD+ addition the fluorescence intensity increased distinctly while the fluorescence enhancement rate was dependent on the employed NAD+ concentration. This phenomenon is illustrated in figure 5 which describes the flourescence increase after normalization of all fluorescence values on the first measured value for each measurement series (NAD+ concentration) from figure 4.
As expected the initial enhancement rate of fluorescence intensity reached almost saturation as soon as using NAD+ concentrations higher then 250 nM which matched the employed molecular beacon concentration. In this case the molecular beacon, the second substrate for LigA, ought to be the limiting factor for the enzymatic reaction, but not the NAD+ which is supposed to be detected. Anyway, there seems to exist a linear dependence for the NAD+ concentration and the initial enhancement rate of fluorescence for NAD+ concentrations below the molecular beacon concentration. This is shown in figure 6 in which the linear dependence is indicated by linear regression of the data. As you can see the NAD+ concentration correlates with the average of fluorescence enhancement rate in 200 s after NAD+ addition (initial velocity) in a linear way as long as the NAD+ concentration was kept below the systems capacity. This means that a specific fluorescence enhancemant rate is characteristic for a particular NAD+ concentration caused by a fixed affinity of LigA for NAD+.

The fluorescence intensity enhancement is supposed to be caused by LigA ligating the split target in a NAD+-dependent manner. This becomes clearly first due to the absence of fluorescence intensity increase for the negative control (0 nM NAD+) and second due to the lengthy fluorescence enhancement after NAD+ addition suggesting enzymatic activity by LigA. This could be directly visualized by taking pictures of the reactions endpoint. As illustrated in figure 7 the fluorescence intensity for added LigA and NAD+ reached nearly the same level as for the positive control (complete target). This was not the case when NAD+ was missing in the reaction mix with LigA and the split target.

Conclusions
LigA, the NAD+-dependent DNA ligase from E. coli, can be utilized for a molecular beacon based bioassay detecting NAD+ in nano molarity scale. It could has been shown that the NAD+ concentration determines the initial fluorescence enhancement rate as a result of ligation of the split target and formation of the molecular beacons open state. While the NAD+ concentration is kept below the molecular beacon concentration there is a linear dependence for both parameters. By varying the molecular beacon concentration the range for linear dependence of NAD+ concentration and initial velocity of LigA should be expandable. Furthermore, the NAD+ concentration might be reduced testing the limit of detection for which the literature value is 0,3 nM NAD+. Finally, LigA seems to be suitable as a model system for bacterial DNA ligases regarding NAD+-dependence due to the highly conserved functional domains throughout the bacterial kingdom. This could be useful in terms of antibiotic drug design utilizing the bacterial DNA ligases specifity for its cofactor NAD+.
References
Gajiwala KS, Pinko C (2004) Structural Rearrangement Accompanying NAD+ Synthesis within a Bacterial DNA Ligase Crystal, [http://www.cell.com/structure/abstract/S0969-2126%2804%2900235-7 Structure 12(8):1449-1459].
Miesel L, Kravec C, Xin AT, McMonagle P, Ma S, Pichardo J, Feld B, Barrabee E, Palermo R (2007) High-throughput assay for the adenylation reaction of bacterial DNA ligase, [http://medical-journals.healia.com/doc/17493575/A-high-throughput-assay-for-the-adenylation-reaction-of-bacterial-DNA-ligase Analytical Biochemistry 366:9-17].
Nandakumar J, Nair PA, Shuman S (2007) Last Stop on the Road to Repair: Structure of E. coli DNA Ligase Bound to Nicked DNA-Adenylate, [http://www.cell.com/molecular-cell/abstract/S1097-2765%2807%2900144-X Molecular Cell 26(2):257-271].
Tang Z, Liu P, Ma C, Yang X, Wang K, Tan W, Lv X (2011) Molecular Beacon Based Bioassay for Highly Sensitive and Selective Detection of Nicotinamide Adenine Dinucleotide and the Activity of Alanine Aminotransferase, [http://pubs.acs.org/doi/abs/10.1021/ac102742k Anal Chem 83(7):2505-2510].
Tyagi S, Kramer FR (1996) Molecular beacons: probes hat fluoresce upon hybridization, [http://www.nature.com/nbt/journal/v14/n3/abs/nbt0396-303.html Nature Biotechnology 14:303-308].
 "
"

