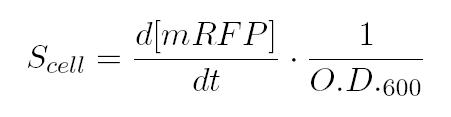|
|
| Line 10: |
Line 10: |
| | | | |
| | <p style="text-align:center;"><span style="font-style:italic;">Signalling is nothing without control...</span></p> | | <p style="text-align:center;"><span style="font-style:italic;">Signalling is nothing without control...</span></p> |
| | + | <br> |
| | | | |
| | | | |
| | + | <table id="toc" class="toc"><tr><td><div id="toctitle"><h2>Contents</h2></div> |
| | + | <ul> |
| | + | <li class="toclevel-1"><a href="#Mathematical_modelling_page"><span class="tocnumber">1</span> <span class="toctext">Mathematicall modeling page</span></a> |
| | + | <ul> |
| | + | <li class="toclevel-2"><a href="#The importance of the mathematical model"><span class="tocnumber">1.1</span> <span class="toctext">The importance of tha mathematical model</span></a></li> |
| | + | <li class="toclevel-2"><a href="#Equations_for_gene_networks"><span class="tocnumber">1.2</span> <span class="toctext">Equations for gene networks</span></a></li> |
| | + | <ul> |
| | + | <li class="toclevel-3"><a href="#Equations_1_and_2"><span class="tocnumber">1.2.1</span> <span class="toctext">Equations (1) and (2)</span></a></li> |
| | + | <li class="toclevel-3"><a href="#Equation_3"><span class="tocnumber">1.2.2</span> <span class="toctext">Equation (3)</span></a></li> |
| | + | <li class="toclevel-3"><a href="#Equation_4"><span class="tocnumber">1.2.3</span> <span class="toctext">Equation (4)</span></a></li> |
| | + | </ul> |
| | + | |
| | + | <li class="toclevel-2"><a href="#Table_of_parameters"><span class="tocnumber">1.3</span> <span class="toctext">Table of parameters</span></a></li> |
| | | | |
| - | <br> | + | <li class="toclevel-2"><a href="#Parameter_estimation"><span class="tocnumber">1.4</span> <span class="toctext">Parameter estimation</span></a></li> |
| | + | <ul> |
| | + | <li class="toclevel-3"><a href="#Ptet_&_Plux"><span class="tocnumber">1.4.1</span><span class="toctext">Ptet & Plux</span></a></li> |
| | + | <li class="toclevel-3"><a href="#AiiA"><span class="tocnumber">1.4.2</span> <span class="toctext">AiiA</span></a></li><li class="toclevel-3"><a href="#LuxI"><span class="tocnumber">1.4.3</span> <span class="toctext">LuxI</span></a></li></ul> |
| | | | |
| - | <p>MODELLING <br><br>
| |
| | | | |
| - | <b>BASIC CONCEPT</b> <br><br> | + | <li class="toclevel-2"><a href="#Simulations"><span class="tocnumber">1.5</span> <span class="toctext">Simulations</span></a></li> |
| - | (se trovo articoli e riferimenti posso cambiare la prima frase dicendo "It is well recognized that...") <br>
| + | |
| - | Synthetic biology greatly relies on modelling as a tool for quantitatively analysing the behaviour of a system. Specifically, it allows to conduct dynamical and stationary phase analyses and parameter sensitivity estimations, based on a mathematical description of the biological system of interest. Therefore it is a valuable predictive tool of the desired behaviour, allowing to test different configurations of the system prior to its implementation. Moreover, it is an essential constituent of the experimental approach, as regards the design of the experiments for the characterization of the parts of the system and data elaboration.<br>
| + | |
| | | | |
| - | According to this, modelling plays a central role in the development of our project, from the characterization of the individual components to the design of the final device.
| + | <li class="toclevel-2"><a href="#References"><span class="tocnumber">1.6</span> <span class="toctext">References</span></a></li> |
| - | <div>Indeed, for every functional part designed, we have realized several variants differing in the entity of the input-output relation. For example, we have assembled various combinations of the promoters of our circuit (namely, pTet and pLux) with different RBSs, resulting in a particular POPSin-POPSout relation.</div><br> | + | </ul> |
| | + | </li> |
| | + | </ul> |
| | + | </li> |
| | + | </ul> |
| | + | </td></tr></table> |
| | + | <br><br> |
| | | | |
| - | "mettere disegno?"<br><br>
| |
| | | | |
| - | After the quantitative characterization of every part, modelling of the final assembled device allows us to predict the global input-output relation of the circuit. This in turn gives an insight into the best choice for the final device, based on criteria such as the achievement of the desired behaviour, the degree of sensitivity and biological tolerance.<br>
| + | <a name="Mathematical_modeling_page"></a><h1><span class="mw-headline"> <b>Mathematical modelling page</b> </span></h1> |
| | + | <div style='text-align:justify'>Mathematical modelling plays nowadays a central role in Synthetic Biology, due to its ability to serve as a crucial link between the concept and realization of a biological circuit. |
| | + | According to this, after a brief overview about the advantages that modelling engineered circuits can bring, we deeply analyze the system of equation formulas, underlining the role and the function of the parameters involved. <br> |
| | + | Then, experimental procedures for parameters estimation are presented and, finally, different types of circuit are discussed and their simulations performed, using ODE's with MATLAB and explainig the difference between a closed-loop model and an open one.</div> |
| | + | <br /> |
| | + | <br /> |
| | | | |
| - | In this section the mathematics of our project is provided: first, the system of equations is introduced, together with an explanation of the variables and parameters involved. Subsequently, experimental procedures for parameters estimation are presented.<br><br>
| |
| - |
| |
| - | <b>SYSTEM OF EQUATIONS Equazioni</b><br><br>
| |
| | | | |
| | | | |
| | + | <a name="The importance of the mathematical model"></a><h2> <span class="mw-headline"> <b>The importance of the mathematical model</b> </span></h2> |
| | + | <div style='text-align:justify'>Several motivations are strong enough to accept the idea that mathematical model is very useful in this study.</div> <br> |
| | + | <ul><li>Firstly in the initial steps of the project, beacuse of its capability to predict the kinetics of the enzymes (aiiA, Luxi) and HSL involved in our gene network, well realizing the <em>a-priori</em> identification in silico in order to understand if the complex circuit's structure and functioning could be achievable.</li></ul> |
| | | | |
| | + | <ul><li>Secondly, for the parametric identification. Using the <em>lsqnonlin</em> function of MATLAB it was possible to get all the parameters involved in the model and consequently to know, for example, the shape of the activation curve of the promoters (Plux, Ptet), according to the <em>a-posteriori</em> identification. </li></ul> |
| | | | |
| | + | <ul><li>Thirdly, the reproducibility. Studing and characterizing simple subparts can allow us not only to predict the behavior of the final circuit, but also it can be useful in other studies, facing with the same basic modules. </li></ul> |
| | + | </p><br> |
| | | | |
| - | "\frac{d[HSL]}{dt}= N*Vmax_L_u_x_I*\frac{LuxI}{(km_L_u_x_I + LuxI)} - N*Vmax_A_i_i_A*\frac{[HSL]}{(km_A_i_i_A + [HSL])} - %gamma * [HSL]"<br><br>
| |
| | | | |
| - | We have condensed in a unique equation transcription and translation processes. (riferimenti ad altri studi).
| |
| - | Equations (1) and (2) have identical structure, differing only in the parameters involved.
| |
| - | <div>The first term describes, through Hill's equation formalism, the synthesis rate of the protein of interest (either LuxI or AiiA) depending on the concentration of the inducible protein (anhydrotetracicline -aTc- or HSL respectively). As can be seen in the parameters table (see below), the term delta stands for the leakage activity of the promoter, who is supposed to be partially active, even in the absence of inducer. In particular, in equation (1), the quite total inhibition of pTet promoter is due to the constitutive production of TetR by our MGZ1 strain, while in equation (2), pLux is almost repressed in the absence of the complex given by LuxR and HSL.</div> <br>
| |
| | | | |
| - | In the first term of equation (2) we have described the inducer as being represented only by HSL. This formalism stems from the fact that our final device offers a constitutive production of LuxR (due to the upstream constitutive promoter pLac), so that, assuming it abundant in the cytoplasm, we can derive the semplification of attributing pLux promoter induction only by HSL.<br>
| + | <a name="Equations_for_gene_networks"></a><h2> <span class="mw-headline"> <b>Equations for gene networks</b> </span></h2> |
| | + | <div style='text-align:justify'><div style='text-align:justify'><div class="thumbinner" style="width: 800px;"><a href="File:Circuito.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/2/2a/Circuito.jpg" class="thumbimage" height="80%" width="80%"></a></div></div> |
| | + | <br> |
| | + | <div style='text-align:justify'><div class="thumbinner" style="width: 850px;"><a href="File:Schema_controllo.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/e/e2/Schema_controllo.jpg" class="thumbimage" height="50%" width="80%"></a></div></div> |
| | + | <br> |
| | + | <div class="center"></div> |
| | + | <div style='text-align:justify'><div class="thumbinner" style="width: 850px;"><a href="File:Model1.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/0/07/Model1.jpg" class="thumbimage" height="95%" width="80%"></a></div></div> |
| | + | <br> |
| | | | |
| - | The second term in equation (1) and (2) is composed of two parts. The first one (gamma*LuxI/HSL) describes with a linear relation the degradation rate per cell of the protein. The second one (mu*(Nmax-N)/Nmax)*luxI/HSL) is a dilution term and is related to the cell replication process. To understand this, let's consider the simplest case of a single cell's division.<br><br>
| |
| | | | |
| - | <b>DISEGNO CELLULA CHE SI DIVIDE</b><br><br> | + | <a name="Equations_1_and_2"></a><h4> <span class="mw-headline"> <b>Equations (1) and (2)</b> </span></h4> |
| | + | <div style='text-align:justify'>We have condensed in a unique equation transcription and translation processes. Equations (1) and (2) have identical structure, differing only in the parameters involved. |
| | + | The first term describes, through Hill's equation formalism, the synthesis rate of the protein of interest (either LuxI or AiiA) depending on the concentration of the inducible protein (anhydrotetracicline -aTc- or HSL respectively). As can be seen in the parameters table (see below),α refers to the maximum activation of the promoter, δ stands for its leakage activity (this means that the promoter is quite induced even if there is no input). In particular, in equation (1), the quite total inhibition of pTet promoter is due to the constitutive production of TetR by our MGZ1 strain, while, in equation (2), Plux is almost repressed in the absence of the complex given by LuxR and HSL.<br> |
| | + | In the first term of equation (2) we have described the inducer as being represented only by HSL. This formalism stems from the fact that our final device offers a constitutive production of LuxR (due to the upstream constitutive promoter pLac), so that, assuming it abundant in the cytoplasm, we can derive the semplification of attributing pLux promoter induction only by HSL: this is the reason why we didn' t consider LuxR in the equations system as well as LuxI and AiiA.<br> |
| | + | Furthermore, in both equations (1) and (2) k and η stands respectively for the other parameter of the Hill relationship. |
| | + | The second term in equation (1) and (2) is composed of two parts. The first one (γ*LuxI/AiiA) describes with a linear relation the degradation rate per cell of the protein. The second one (μ*(Nmax-N)/Nmax)*LuxI/AiiA) takes into account the dilution term and is related to the cell replication process. |
| | + | </div> |
| | + | <br> |
| | | | |
| - | When this happens, we can assume that all the content of the mother cell equally distributes in the two derived cells. Consequently, if we had for example ten molecules of LuxI per cell, after the cellular division they would become five molecules per cell.<br>
| + | <a name="Equation_3"></a><h4> <span class="mw-headline"> <b>Equation (3)</b> </span></h4> |
| | + | <div style='text-align:justify'>The processes described here are not those of transcription and translation, but in principle are enzymatic reactions either related to the production or the degradation of HSL. Based on the experiments performed, we derived Hill's equation in the case of η=1. They cannot be exactly defined Michaelis Menten's equations since that in our formalism, LuxI and AiiA aren't described as enzymes (since they appear also in the denominator). We simply derived empirical formulas relating either LuxI or AiiA to HSL, and treated them with the typical Michaelis Menten formalism since they presented the corresponding sigmoidal shape/switching like behaviour. Regarding to this, we believe that the saturation phenomenon observed either in HSL production rate due to LuxI, or HSL degradation rate due to AiiA, underlies limiting elements in cell metabolism. |
| | + | In the cell HSL binds to LuxR, and two HSL molecules form a tetramer with two complementary LuxR molecules to form a complex which can then activate the promoter Plux.<br> |
| | + | Intuitively, LuxI activity as an enzyme encounters an intrinsic limit in HSL synthesis depending on the finite and hypothetically fixed substrate concentration (namely SAM and hexanoyl-ACP, see ref.); this means that at a certain LuxI concentration, all the substrate forms activation complexes with LuxI, so that there is no more substrate available for the other LuxI produced. HSL degradation rate is limited by its availability; even if the concentration varies with time, there is always a corresponding limit in AiiA concentration, which determines a saturation in the degradation rate.<br> |
| | + | Moreover, both the formulas relating either LuxI or AiiA to HSL are multiplied by the number of cells N, due to the property of the lactone to diffuse free inside/outside bacteria. |
| | + | The third term in equation (3) is similar to the corresponding ones present in the first two equations and describes the intrinsic protein degradation.</div> |
| | + | <br> |
| | + | <a name="Equation_4"></a><h4> <span class="mw-headline"> <b>Equation (4)</b> </span></h4> |
| | + | <div style='text-align:justify'>Equation (4) is the common equation describing logistic cell growth, depending on the rate μ and the maximum number N<sub>MAX</sub> of cells per well reachable.</div> |
| | + | <br><br> |
| | | | |
| - | Now consider equation (3). The processes described herein are not those of transcription and translation, but in principle are enzymatic reactions either related to the production or the degradation of HSL. Based on the experiments performed, we derived Hill's equation in the case of eta=1. They cannot be exactly defined Michaelis Menten's equations since that in our formalism, LuxI and AiiA aren't described as enzymes (since they appear also in the denominator). We simply derived empirical formulas relating either LuxI or AiiA to HSL, and treated them with the typical Michaelis Menten formalism since they presented the corresponding sigmoidal shape/switching like behaviour. Regarding to this, we believe that the saturation phenomenon observed either in HSL production rate due to LuxI, or HSL degradation rate due to AiiA, underlies limiting elements in cell metabolism. Intuitively, LuxI activity as an enzyme encounters an intrinsic limit in HSL synthesis depending on the finite and hypothetically fixed substrate concentration (namely SAM and hexanoyl-ACP, see ref.); this means that at a certain LuxI concentration, all the substrate forms activation complexes with LuxI, so that there is no more substrate available for the other LuxI produced.<br>
| |
| - | <div>Similarly, HSL degradation rate is limited by HSL availability; even if HSL concentration varies with time, there is always a corresponding limit in AiiA concentration, which determines a saturation in the degradation rate.</div><br>
| |
| | | | |
| - | The third term in equation three is similar to the corresponding ones present in the first two equations and describes protein degradation.<br><br>
| + | <a name="Table_of_parameters"></a><h2> <span class="mw-headline"> <b>Table of parameters</b> </span></h2> |
| - | | + | <br> |
| - | Equation (4) is the common equation describing logistic cell growth.<br><br>
| + | |
| | | | |
| | <center> | | <center> |
| Line 61: |
Line 103: |
| | <tr> | | <tr> |
| | <td class="row"><b>Parameter</b></td> | | <td class="row"><b>Parameter</b></td> |
| | + | <td class="row"><b>Description</b></td> |
| | <td class="row"><b>Unit of Measurement</b></td> | | <td class="row"><b>Unit of Measurement</b></td> |
| | <td class="row"><b>Value</b></td> | | <td class="row"><b>Value</b></td> |
| Line 67: |
Line 110: |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">α<sub>pTet</sub></td> | + | <td class="row">α<sub>P<sub>Tet</sub></sub></td> |
| - | <td class="row">[(mRFP/min)/cell]</td> | + | <td class="row">maximum transcription rate of Ptet</td> |
| | + | <td class="row">[(AUr/min)/cell]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| Line 74: |
Line 118: |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">δ<sub>pTet</sub></td> | + | <td class="row">δ<sub>P<sub>Tet</sub></sub></td> |
| | + | <td class="row">leakage factor of promoter Ptet basic activity</td> |
| | <td class="row">[-]</td> | | <td class="row">[-]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 80: |
Line 125: |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">k<sub>pTet</sub></td> | + | <td class="row">η<sub>P<sub>Tet</sub></sub></td> |
| - | <td class="row">[nM]</td> | + | <td class="row">Hill coefficient of Ptet</td> |
| | + | <td class="row">[-]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">η<sub>pTet</sub></td> | + | <td class="row">k<sub>P<sub>Tet</sub></sub></td> |
| - | <td class="row">[-]</td> | + | <td class="row">dissociation costant of Ptet ? </td> |
| | + | <td class="row">[nM]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">γ<sub>pTet</sub></td> | + | <td class="row">α<sub>P<sub>Lux</sub></sub></td> |
| - | <td class="row">[1/min]</td> | + | <td class="row">maximum transcription rate of Plux</td> |
| | + | <td class="row">[(AUr/min)/cell]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| | + | |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">α<sub>pLux</sub></td> | + | <td class="row">δ<sub>P<sub>Lux</sub></sub></td> |
| - | <td class="row">[(mRFP/min)/cell]</td> | + | <td class="row">leakage factor of promoter Plux basic activity</td> |
| | + | <td class="row">[-]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| - |
| |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">δ<sub>pLux</sub></td> | + | <td class="row">η<sub>P<sub>Lux</sub></sub></td> |
| | + | <td class="row">Hill coefficient of Plux</td> |
| | <td class="row">[-]</td> | | <td class="row">[-]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 111: |
Line 161: |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">k<sub>pLux</sub></td> | + | <td class="row">k<sub>P<sub>Lux</sub></sub></td> |
| | + | <td class="row">dissociation costant of Plux ?</td> |
| | <td class="row">[nM]</td> | | <td class="row">[nM]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 117: |
Line 168: |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">η<sub>pLux</sub></td> | + | <td class="row">γ<sub>P<sub>Lux</sub></sub></td> |
| - | <td class="row">[-]</td> | + | <td class="row">LuxI costant degradation</td> |
| | + | <td class="row">[1/min]</td> |
| | + | <td class="row">-</td> |
| | + | </tr> |
| | + | <tr> |
| | + | <td class="row">γ<sub>AiiA</sub></td> |
| | + | <td class="row">AiiA costant degradation</td> |
| | + | <td class="row">[1/min]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">γ<sub>pLux</sub></td> | + | <td class="row">γ<sub>HSL</sub></td> |
| | + | <td class="row">HSL costant degradation</td> |
| | <td class="row">[1/min]</td> | | <td class="row">[1/min]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 130: |
Line 189: |
| | <tr> | | <tr> |
| | <td class="row">V<sub>max_LuxI</sub></td> | | <td class="row">V<sub>max_LuxI</sub></td> |
| | + | <td class="row">maximum transcription rate of LuxI</td> |
| | <td class="row">[nM/(min*cell)]</td> | | <td class="row">[nM/(min*cell)]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 136: |
Line 196: |
| | <tr> | | <tr> |
| | <td class="row">k<sub>m_LuxI</sub></td> | | <td class="row">k<sub>m_LuxI</sub></td> |
| - | <td class="row">[nM]</td> | + | <td class="row">dissociation costant ?</td> |
| | + | <td class="row">[AUr/cell]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| - | <td class="row">V<sub>max_AiiA</sub></td> | + | <td class="row">k<sub>CAT</sub></td> |
| - | <td class="row">[nM/(min*cell)]</td> | + | <td class="row"> ?? </td> |
| | + | <td class="row">[1/(min*cell)]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| Line 147: |
Line 209: |
| | <tr> | | <tr> |
| | <td class="row">k<sub>m_AiiA</sub></td> | | <td class="row">k<sub>m_AiiA</sub></td> |
| - | <td class="row">[nM]</td> | + | <td class="row">dissociation costant ?</td> |
| - | <td class="row">-</td>
| + | <td class="row">[AUr/cell]</td> |
| - | </tr>
| + | |
| - | | + | |
| - | <tr>
| + | |
| - | <td class="row">γ<sub>HSL</sub></td>
| + | |
| - | <td class="row">[1/min]</td> | + | |
| | <td class="row">-</td> | | <td class="row">-</td> |
| | </tr> | | </tr> |
| | | | |
| | <tr> | | <tr> |
| - | <td class="row">N<sub>max</sub></td> | + | <td class="row">N<sub>MAX</sub></td> |
| | + | <td class="row">maximum number of bacteria per well</td> |
| | <td class="row">[cell]</td> | | <td class="row">[cell]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 165: |
Line 223: |
| | <tr> | | <tr> |
| | <td class="row">μ</td> | | <td class="row">μ</td> |
| | + | <td class="row">rate of bacteria groth</td> |
| | <td class="row">[1/min]</td> | | <td class="row">[1/min]</td> |
| | <td class="row">-</td> | | <td class="row">-</td> |
| Line 170: |
Line 229: |
| | | | |
| | </table> | | </table> |
| - | </center> | + | </center> |
| | | | |
| - | <br><br><b>Spiegazione parametri e test con cui misurarli</b><br><br> | + | <br><div style='text-align:justify'>According to the table above, the unit of the state variables are:</div><br> |
| - | | + | |
| - | <b>Equations (1) and (2)</b><br><br>
| + | |
| - | In this section we examine the parameters of the model and justify the units of measure, relating them to the experiments performed for the characterization of the parts.<br>
| + | |
| - | | + | |
| - | Relating to the equations (1) and (2), we assume acquainted the protein degradation rate, which equals gamma_i=0.0173 due to the presence of the LVA tag (see registry...).<br>
| + | |
| - | | + | |
| - | Moreover the dilution term is exactly the specific growth rate, to be determined through an apposite experiment.<br>
| + | |
| - | | + | |
| - | What remains is the term describing the synthesis rate.
| + | |
| - | <div>The parameters involved were experimentally determined through ad hoc designed experiments. We exploited specifically assembled parts, formed by the sequence of <br><br> | + | |
| - | | + | |
| - | promoter-RBS-mRFP-TT FARE DISEGNO AL RIGUARDO<br><br>
| + | |
| - | | + | |
| - | Indeed, this parts are also amenable to be applied as components for subsequent iGem projects. </div><br>
| + | |
| - | | + | |
| - | What we want to characterize is the promoter+RBS complex. We realize this by introducing the mRFP fuorescent protein (followed by a double terminator), and we make the assumption that the number of fluorescent protein produced is exactly the same as the number given by any other protein that would be espressed instead of the mRFP. In other words, in our hypotesis, if we would substitute the mRFP coding region with a region coding for another protein, we would obtain the same synthesis rate. Clearly this is a strong hypotesis, however its level of approximation is considered to be adequate.
| + | |
| - | <div>The construct realized is the best suited for our laboratory equipment instruments. Specifically, our TECAN spectrophotometer allows us to measure Scell(dmRFP/dt/OD) as a function of inducer concentration, thereby providing the desired input-output relation (inducer concentration versus promoter+RBS activity), which was modelled as a Hill curve.</div><br>
| + | |
| - | | + | |
| - | alfa_pTet and alfa_pLux represent the protein maximum synthesis rate, which is reached, in accordance with Hill's formalism, when the inducer concentration tends to infinite, and, more practically, for sufficently high concentrations of inducer.<br> <br>
| + | |
| - | | + | |
| - | delta_ptet and delta_plux, as previously explained, are responsible for basal protein expression (given by alfa_pTet*delta_pTet/pLux), liable for protein production (LuxI and AiiA respectively) even in the absence of autoinducer.<br><br>
| + | |
| - | | + | |
| - | eta_ptet and eta_plux are thed Hill's cooperativity constants, and determine the ripidity of the switch like curve relating Scell with the concentration of inducer.<br>
| + | |
| - | <div>Katc and kplux are the semi-saturation constant, and in case of a unity value for eta_atc and eta_plux, indicate the concentration of substrate at which half the synthesis rate is achieved.</div><br>
| + | |
| - | | + | |
| - | The unities of the various parameters can be easily derived considering the hill equation and the unity of its left handed side.<br><br>
| + | |
| - | | + | |
| - | [dGFP/dt/cell]=[dGFP/dt/cell]*([-]+([.]-[-])/([-]+[nM]/[nM]))<br><br>
| + | |
| - | | + | |
| - | <b>Third equation</b><br>
| + | |
| - | <br>
| + | |
| - | It can be recognized that the characterization of the processes implied in this equation required a greatest level application and formalization, since it involved us to design ad hoc experimental measures we hadn't previously engaged in. Both the experiments can be regarded as made of tho phases, which we can define the <b>stimulation/inducing phase</b> and the <b>reading phase</b> respectively. Each of them relies on a specific construct appropiately induced; the inducing phase construct is different for LuxI dependent HSL synthesis rate and AiiA dependent HSL degradation rate characterization, while the reading phase construct is the same for the two experiments; indeed, it relies on T9002, and allows to determine HSL concentration based on the Scell produced Further details are given in the following paragraphs.<br><br>
| + | |
| - | | + | |
| - | <b>LuxI dependent HSL synthesis rate</b><br>
| + | |
| - | <div>As we have previously seen, the first term in equation (3) describes HSL synthesis rate dependence on LuxI concentration. We have also yet explained why we took such a formalism. This is not the typical mathematical modelling approach used to describe pLux promoter activity, usually represented as a function of HSL-LuxR complex concentration. There is plenty of literature references in this context, while little is available in the case of LuxR abundance. Theoretically, this is a particular situation of the more general relating to the complex contemplation, but obviously it is not easy to derive from it.</div>
| + | |
| - | In the stimulation phase of the experimental set up, we used the following construct:<br><br>
| + | |
| - | | + | |
| - | pTet-RBS-LuxI-TT<br><br>
| + | |
| - | | + | |
| - | Theoretically, the biological processes related to this biobrick are those of LuxI synthesis and degradation and the LuxI driven HSL synthesis. In order to isolate the last one, namely HSL synthesis, we should make sure that it is possible to consider LuxI in stationary phase (and so at a constant concentration) and to leave aside LuxI degradation.
| + | |
| - | <div>The condition on LuxI degradation subsists, since we operate on a shorter time scale (order of hours).
| + | |
| - | Moreover, the adopted experimental protocol allowed us to consider LuxI in stationary phase.
| + | |
| - | So we can hypotesize that the HSL produced only depends on the relation between LuxI and HSL, given a specific stationary concentration of LuxI, that in turn depends on aTc concentration.</div>
| + | |
| - | In order to measure this HSL concentration, we used the T9002 device (reading phase).<br>
| + | |
| - | | + | |
| - | It is worth mentioning that we can predict LuxI "concentration" (in terms of (dmRFP/dt)/OD) based on the previously characterized pTet-RBS constructs. Moreover, varying the RBS, we can span a relatively large range of LuxI concentrations, providing us with more experimental points.<br>
| + | |
| - | | + | |
| - | Hereafter the single passes of the experiment are schematically proposed, in order to better understand it:<br><br>
| + | |
| - | | + | |
| - | <div>1) Transform a MGZ1 E. coli strain with the pTet-RBS-LuxI-TT construct, and wait three hours for reaching the exponential phase growth.</div>
| + | |
| - | <div>2) Induce the culture with a proper amount of aTc.</div>
| + | |
| - | <div>3) Take samples of the supernatant at different times (i.e. 0 h, 1 h, 4 h) and store them in the freezer at -20° C </div>
| + | |
| - | <div>4) Retrieve the supernatants prepared and use them to induce the T9002 constructs contained in the TECAN spectrophotometer wells</div>
| + | |
| - | <div>5) Wait until sensing is completed and retrieve the results from TECAN.</div><br>
| + | |
| - | | + | |
| - | <b>AiiA dependent HSL degradation rate</b><br>
| + | |
| - | AiiA dependent HSL degradation rate experiment is the same as the previous one as regards the passes involved, and simply differs in that there is no part producing HSL, so we have to inject it at a proper concentration. Then, the construct used in the stimulation phase, namely<br>
| + | |
| - | | + | |
| - | pTet-RBS-AiiA-TT,<br>
| + | |
| - | | + | |
| - | brings to the production of a known amount of AiiA (expressed in terms of dmRFP/dt/cell). Therefore, this time, when we take samples at different times from aTc induction (after having waited for AiiA to become in stationary phase) we will see AiiA dependent HSL degradation.<br>
| + | |
| - | | + | |
| - | So the following are the passes involved in the experiment:<br><br>
| + | |
| - | | + | |
| - | <div>1) Transform a MGZ1 E. coli strain with the pTet-RBS-AiiA-TT construct, and wait three hours for reaching the exponential phase growth.</div>
| + | |
| - | <div>2) Induce the culture with a proper amount of aTc.</div>
| + | |
| - | <div>3) Take samples of the supernatant at different times (i.e. 0 h, 1 h, 4 h) and store them in the freezer at -20° C </div>
| + | |
| - | <div>4) Retrieve the supernatants prepared and use them to induce the T9002 constructs contained in the TECAN spectrophotometer wells</div>
| + | |
| - | <div>5) Wait until sensing is completed and retrieve the results from TECAN.</div><br>
| + | |
| - | | + | |
| - | <b>Equation (4)</b><br><br>
| + | |
| - | The parameters N<sub>max</sub> and μ, can be calculated from the analysis of the OD<sub>600</sub> produced by our MGZ1 culture. In particular, μ is derived as the slope of the log(OD<sub>600</sub>) growth curve. N<sub>max</sub> is determined with a proper procedure. After having reached saturation phase and having retrieved the corresponding OD<sub>600</sub>, we take a sample of the culture and make serial dilutions of it, then we plate the final diluted culture on a Petri and wait for the formation of colonies. The dilution serves to avoid the growth of too many and too close colonies in the Petri. Finally, we count the number of colonies, which correspond to N<sub>max</sub>.<br><br>
| + | |
| - | | + | |
| - | <font size="3"><b>CLOSED LOOP VS OPEN LOOP</b></font><br><br>
| + | |
| - | Now that we have gone deep into the various aspects of the mathematical model of our closed loop, it's time to explain why it is advantageous with respect to the open loop.<br>
| + | |
| - | | + | |
| - | In order to see that, we implemented and simulated in Matlab our closed loop circuit and the open loop one, consisting of the same construct without the feedback loop, that is the part pLux-RBS-AiiA-TT (E37-E40). The table below provides the values for the parameters of the model.<br><br>
| + | |
| | | | |
| | <center> | | <center> |
| | <table class="data"> | | <table class="data"> |
| | <tr> | | <tr> |
| - | <td class="row"><b>Parameter</b></td> | + | <td class="row"><b>State variable</b></td> |
| | <td class="row"><b>Unit of Measurement</b></td> | | <td class="row"><b>Unit of Measurement</b></td> |
| - | <td class="row"><b>Value</b></td>
| + | </tr> |
| - | </tr>
| + | |
| | | | |
| | + | <tr> |
| | + | <td class="row"><sup>d[LuxI]</sup>⁄<sub>dt</sub></td> |
| | + | <td class="row">[<sup>AUr</sup>⁄<sub>(min*cell)</sub>]</td> |
| | + | </tr> |
| | | | |
| - | <tr>
| + | <tr> |
| - | <td class="row">α<sub>pTet</sub></td>
| + | <td class="row"><sup>d[AiiA]</sup>⁄<sub>dt</sub></td> |
| - | <td class="row">[(mRFP/min)/cell]</td>
| + | <td class="row">[<sup>AUr</sup>⁄<sub>(min*cell)</sub>]</td> |
| - | <td class="row">-</td>
| + | </tr> |
| - | </tr>
| + | |
| | | | |
| | + | <tr> |
| | + | <td class="row"><sup>d[HSL]</sup>⁄<sub>dt</sub></td> |
| | + | <td class="row">[<sup>nM</sup>⁄<sub>(min)</sub>]</td> |
| | + | </tr> |
| | | | |
| - | <tr>
| + | <tr> |
| - | <td class="row">δ<sub>pTet</sub></td>
| + | <td class="row"><sup>d[N]</sup>⁄<sub>dt</sub></td> |
| - | <td class="row">[-]</td>
| + | <td class="row">[<sup>cell</sup>⁄<sub>(min)</sub>]</td> |
| - | <td class="row">-</td>
| + | </tr> |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| + | </table><br> |
| - | <td class="row">k<sub>pTet</sub></td>
| + | |
| - | <td class="row">[nM]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| |
| - | <td class="row">η<sub>pTet</sub></td>
| |
| - | <td class="row">[-]</td>
| |
| - | <td class="row">-</td>
| |
| - | </tr>
| |
| | | | |
| - | <tr>
| |
| - | <td class="row">γ<sub>pTet</sub></td>
| |
| - | <td class="row">[1/min]</td>
| |
| - | <td class="row">-</td>
| |
| - | </tr>
| |
| | | | |
| - | <tr>
| + | <a name="Parameter_estimation"></a><h2> <span class="mw-headline"> <b>Parameter estimation</b></span></h2><br> |
| - | <td class="row">α<sub>pLux</sub></td>
| + | |
| - | <td class="row">[(mRFP/min)/cell]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| | + | <div style='text-align:justify'>In this section we examine the parameters of the model and justify the units of measure, relating them to the experiments performed for the characterization of the parts. We want to underline again our concept of modelling: beginning to caractherize simplier parts, we get their parameters and we try to predict the behaviour of the final engineerd closed-loop.</div> |
| | | | |
| - | <tr>
| |
| - | <td class="row">δ<sub>pLux</sub></td>
| |
| - | <td class="row">[-]</td>
| |
| - | <td class="row">-</td>
| |
| - | </tr>
| |
| | | | |
| - | <tr>
| + | <a name="Ptet_&_Plux"></a><h4> <span class="mw-headline"> <b>Promoter (Ptet & Plux)</b> </span></h4> |
| - | <td class="row">k<sub>pLux</sub></td>
| + | <div align="center"><div class="thumbinner" style="width: 500px;"><a href="File:Ptet.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/f/f0/Ptet.jpg" class="thumbimage" height="35%" width="50%"></a></div></div> |
| - | <td class="row">[nM]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| + | <div style='text-align:justify'>These are the first subparts tested. |
| - | <td class="row">η<sub>pLux</sub></td>
| + | Firstly, in the figure above "RBSx" stands for, respectively, |
| - | <td class="row">[-]</td>
| + | <a href="http://partsregistry.org/Part:BBa_B0030">RBS30</a>, |
| - | <td class="row">-</td>
| + | <a href="http://partsregistry.org/Part:BBa_B0031">RBS31</a>, |
| - | </tr>
| + | <a href="http://partsregistry.org/Part:BBa_B0032">RBS32</a>, |
| | + | <a href="http://partsregistry.org/Part:BBa_B0034">RBS34</a>. So we get 4 biobricks for each promoter, in order to investigate what happens in different conditions of RBS's efficiency. In this phase of the project we aim to increase our knowledge about promoter Ptet and Plux but it must be said that, here, it' s quite impossible to focus separately on the only activity of the promoter and RBSx; for this reason, when we "characterize promoters", we mean promoter and RBS together. |
| | + | We realize this by introducing the mRFP fuorescent protein (followed by a double terminator), and we make the assumption that the number of fluorescent protein produced, due to the concentration of induction (aTc, HSL for Ptet, Plux respectively) is exactly the same as the number given by any other protein that would be expressed instead of the mRFP. In other words, in our hypotesis, if we would substitute the mRFP coding region with a region coding for another protein, we would obtain the same synthesis rate: this is the reason why the strength of the complex promoter-RBSx is expressed in Arbitrary Units [AUr]. Clearly this is a strong hypotesis, however its level of approximation is considered to be adequate. |
| | + | <br> |
| | + | |
| | + | Keeping this idea in mind, let's talk about the steps to estimate parameters.<br> |
| | + | As shown in the box below, we consider a wide (more or less, depending on the type of test) range of induction and we monitor, during the time, absorbance (line1, line2) and fluorescence (line3); the two vertical segments for each figure highlight the exponential phase of bacteria' s groth. We are able to make these measurement due to the Tecan Infinite F200, spectrophotometer that allows to know the Scell (explained few lines below) as a function of inducer concentration, thereby providing the desired input-output relation (inducer concentration versus promoter+RBS activity), which was modelled as a Hill curve. |
| | + | <br> |
| | + | After that, we can calculate the <em>Scell</em> as: |
| | | | |
| - | <tr>
| + | <div align="center"><div class="thumbinner" style="width: 600px;"><a href="File:Scell.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/5/58/Scell.jpg" class="thumbimage" height="80%" width="45%"></a></div></div> |
| - | <td class="row">γ<sub>pLux</sub></td>
| + | In the end, plotting Scell VS induction, we obtain the activation Hill curve of the promoter considered. |
| - | <td class="row">[1/min]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| + | <div style='text-align:justify'><div class="thumbinner" style="width: 600px;"><a href="File:Box1_new.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/7/71/Box1_new.jpg" class="thumbimage" height="100%" width="120%"></a></div></div> |
| - | <td class="row">V<sub>max_LuxI</sub></td>
| + | |
| - | <td class="row">[nM/(min*cell)]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| + | As shown in the box above, α as already mentioned, represent the protein maximum synthesis rate, which is reached, in accordance with Hill's formalism, when the inducer concentration tends to infinite, and, more practically, for sufficently high concentrations of inducer, meanwhile the product α*δ stands for the leakage activity (induction=0ng/µL), liable for protein production (LuxI and AiiA respectively) even in the absence of autoinducer. The paramenter η is the Hill's cooperativity constant and it affects the rapidity and ripidity of the switch like curve relating Scell with the concentration of inducer. |
| - | <td class="row">k<sub>m_LuxI</sub></td>
| + | Lastly, k stands for the semi-saturation constant and, in case of a unity value for η, it indicates the concentration of substrate at which half the synthesis rate is achieved. |
| - | <td class="row">[nM]</td>
| + | The unities of the various parameters can be easily derived considering the hill equation and the unity of its left handed side (for more details see the <a href="#Table_of_parameters"><span class="toctext">Table of parameters</span></a> above). <br> <br> |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| - | <tr>
| + | |
| - | <td class="row">V<sub>max_AiiA</sub></td>
| + | |
| - | <td class="row">[nM/(min*cell)]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| |
| - | <td class="row">k<sub>m_AiiA</sub></td>
| |
| - | <td class="row">[nM]</td>
| |
| - | <td class="row">-</td>
| |
| - | </tr>
| |
| | | | |
| - | <tr>
| |
| - | <td class="row">γ<sub>HSL</sub></td>
| |
| - | <td class="row">[1/min]</td>
| |
| - | <td class="row">-</td>
| |
| - | </tr>
| |
| | | | |
| - | <tr>
| + | <a name="AiiA"></a><h4> <span class="mw-headline"> <b>AiiA</b> </span></h4> |
| - | <td class="row">N<sub>max</sub></td>
| + | <div align="center"><div class="thumbinner" style="width: 500px;"><a href="File:AiiA.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/3/3e/AiiA.jpg" class="thumbimage" height="26%" width="50%"></a></div></div> |
| - | <td class="row">[cell]</td>
| + | |
| - | <td class="row">-</td>
| + | |
| - | </tr>
| + | |
| | | | |
| - | <tr>
| + | <div style='text-align:justify'> These experiments aims to learn approximately the degradation rate of HSL due to the expression of AiiA. In these case, we are able to quantify exactly the concentration of HSL, using the well-characterized part <a href="http://partsregistry.org/Part:BBa_T9002">BBa_T9002</a> in the previous iGEM. |
| - | <td class="row">μ</td>
| + | <div align="center"><div class="thumbinner" style="width: 500px;"><a href="File:T9002.jpg" class="image"><img alt="" src="https://static.igem.org/mediawiki/2011/c/c2/T9002.jpg" class="thumbimage" height="100%" width="110%"></a></div></div> |
| - | <td class="row">[1/min]</td>
| + | This biobrick receives in input HSL concentration, and returns in output the intensity of fluorescence. |
| - | <td class="row">-</td>
| + | So, our idea is to control the degradation of HSL reading the fluorescence of T9002 due to a certain concentration of HSL; monitoring it in precise samples of time since aTc induction (after having waited enough for AiiA to become in stationary phase), we can estimate the degradation rate, also compared with other constructs, which would't degradate it. According to this, it' s necessary to know very well the reationship input-output of the biosensor: a curve of "calibration" of T9002 is obtain for each test performed, even if, in theory, it should be always the same. |
| - | </tr>
| + | Summarizing in few points, the following are the passes involved in the experiment: |
| | | | |
| - | </table> | + | <ol><li>Transform a MGZ1 E. coli strain with the pTet-RBS-AiiA-TT construct, and wait three hours for reaching the exponential phase growth.</li> |
| - | </center> | + | <li>Induce the culture with a proper amount of aTc.</li> |
| | + | <li>Take samples of the supernatant at different times (i.e. 0 h,1 h,4 h) and store them in the freezer at -20°C</li> |
| | + | <li>Retrieve the supernatants prepared and use them to induce the T9002 construct contained in the TECAN spectrophotometer wells</li> |
| | + | <li>Wait until sensing is completed and retrieve the results from TECAN.</li></ol> |
| | | | |
| - | </p> | + | <div style='text-align:justify'><div class="thumbinner" style="width: 500px;"><a href="" class="image"><img alt="File:Degradation.jpg" src="https://static.igem.org/mediawiki/2011/9/99/Degradation.jpg" class="thumbimage" height="90%" width="140%"></a></div></div><br><br> |
| | | | |
| - | <p> | + | <a name="LuxI"></a><h4> <span class="mw-headline"> <b>LuxI</b> </span></h4> |
| - | The following graphs represent the corresponding results from the two models. As can be seen, both reach a stable equilibrium point, since that there isn't any positive feedback loop capable of bringing instability. However, starting from the same initial conditions (and with the same values for the parameters), the closed loop settles to a HSL steady state level far lower than the open loop, highlighting its capability to limit HSL concentration to a treshold level.
| + | <br> |
| - | </p> | + | <a name="Simulations"></a><h1><span class="mw-headline"> <b>Simulations</b> </span></h1> |
| - | | + | <br> |
| - | | + | <a name="References"></a><h1><span class="mw-headline"> <b>References</b> </span></h1> |
| - | <p> | + | |
| - | INSERISCI GRAFICI
| + | |
| - | </p> | + | |
| - | | + | |
| - | <p> | + | |
| - | On the same basis, it is interesting to observe what happens if we introduce a HSL impulse/stimulus, regarded as a noise. In the open loop model, it adds to the steady state bringing to a higher value of the equilibrium point. On the contrary, the feedback loop circuit is able to partially counteract it and to avoid great changes in the steady state value.
| + | |
| - | </p> | + | |
| - | | + | |
| - | <div class="center"><div class="thumb tnone"><div class="thumbinner" style="width: 700px;"><a href="File:closed_loop.jpg" class="image"><img alt="" class="thumbimage" height="80%" width="80%"></a></div></div></div> | + | |
| | | | |
| - | <p>
| |
| - | INSERISCI GRAFICI
| |
| - | </p>
| |
| | | | |
| - | <p>
| |
| - | On a biological level, the ability to control the concentration of a given molecule reveals fundamental in limiting the metabolic burden of the cell; moreover, in the particular case of HSL signalling molecules, this would give the possibility to regulate quorum sensing based population's behaviours.
| |
| - | </p>
| |
| | | | |
| - | <h4>T9002</h4>
| + | </div> |
| - | <div>
| + | </div> |
| - | T9002 is an input/output device (vedere nel registry), with HSL as input and GFP
| + | |
| - | (more precisely SCell) as output. It was created by (team). With T9002 it is
| + | |
| - | possible to measure the HSL concentration of a substance based on the measured fluorescence
| + | |
| - | intensity.
| + | |
| - | </div>
| + | |
| - | <b>HOW DOES IT WORK </b>
| + | |
| - | <div>
| + | |
| - | First, it is necessary to create the (curva di taratura), which represents the input/output
| + | |
| - | relationship between HSL and SCell. It is obtained by inducing T9002 with known
| + | |
| - | concentrations of HSL, and measuring the corresponding level of GFP intensity. With
| + | |
| - | these experimental values it is possible to derive the non linear least square fitting
| + | |
| - | curve relating HSL to GFP. After that, reading on the (curva di taratura) the GFP
| + | |
| - | level of a solution with unknown HSL concentration, you can get the corresponding
| + | |
| - | HSL level (see figure below).
| + | |
| - | </div>
| + | |
| - | <div>
| + | |
| - | <b>WHY WE USED IT </b>
| + | |
| - | </div>
| + | |
| - | <div>
| + | |
| - | We used T9002 in order to experimentally characterize two processes related to HSL.
| + | |
| - | One is HSL degradation rate dependence on AiiA concentration and the other is HSL
| + | |
| - | synthesis rate variation depending on LuxI concentration.
| + | |
| - | </div>
| + | |
| - | <h3>Equation (3): AiiA DEPENDENT HSL DEGRADATION</h3>
| + | |
| - | <div>
| + | |
| - | <b>STATE OF THE ART </b>
| + | |
| - | </div>
| + | |
| - | As described in (Motivations), AiiA is an enzyme family capable of degrading N-Acyl
| + | |
| - | homoserine lactones (HSLs). Several studies and related papers describe its structural
| + | |
| - | properties and in vitro kinetics (Dong et al 1999, Lee et al 2002, Pan et al 2007,
| + | |
| - | Kim et al 2005, Liu et al 2008, Wang et al 2004, Momb et al 2008). Here, AiiA enzymatic
| + | |
| - | degradation of lactones is described with the common Michaelis Menten equation.<br>On
| + | |
| - | the contrary there are few examples of its application in synthetic biology. Nevertheless
| + | |
| - | Danino et al 2010, in their synchronized quorum of genetic clocks, exploited the
| + | |
| - | quorum sensing signalling molecules (HSL and LuxR) and the quorum quenching lactonase
| + | |
| - | (AiiA) in order to synchronize on a population level the single cell oscillatory
| + | |
| - | behaviour. In the equation relating to internal HSL concentration, they described
| + | |
| - | AiiA dependent HSL degradation with a saturation kinetic.
| + | |
| - | <div>
| + | |
| - | <b>MODELLING OF HSL DEGRADATION BY AiiA </b>
| + | |
| - | </div>
| + | |
| - | <div>
| + | |
| - | We created an ad hoc experimental set up in order to characterize AiiA driven degradation
| + | |
| - | of HSL (see also Measurements). First, we used E37, E38, E39 and E40 AiiA producing
| + | |
| - | constructs induced with aTc and in presence of 1 μM of HSL. <br>Then, we took
| + | |
| - | diluted samples at differing time scales and determined HSL degradation over time
| + | |
| - | through T9002. The adopted mathematical formalism is the saturation kinetic presented
| + | |
| - | as second term in equation 3.
| + | |
| - | </div>
| + | |
| - | <h4>Equation (3): LuxI DEPENDENT HSL SYNTHESIS</h4>
| + | |
| - | <div>
| + | |
| - | <b>MODELLING OF HSL DEGRADATION BY LuxI</b>
| + | |
| - | </div>
| + | |
| - | There is plenty of literature references and synthetic biology models describing
| + | |
| - | LuxI driven HSL synthesis (Danino et al 2010, Goriachev et al 2005, Garcia-Ojalvo
| + | |
| - | et al 2004). Nevertheless, they adopted different mathematical formalisms and they
| + | |
| - | are difficult to apply to our biological system. Similarly to AiiA, we created an
| + | |
| - | ad hoc experimental set up in order to characterize LuxI driven HSL synthesis (see
| + | |
| - | also Measurements). First, we used E28, E31, E41 and E42 LuxI producing constructs
| + | |
| - | induced with aTc. <br>Then, we took diluted samples at differing time scales and
| + | |
| - | determined HSL synthesis over time through T9002. The adopted mathematical formalism
| + | |
| - | is the saturation kinetic presented as first term in equation 3. As can be seen,
| + | |
| - | it is very similar to the one used to describe the degradation of HSL due to AiiA.
| + | |
| - | <h4>MODELLING OF CELL'S POPULATION GROWTH</h4>
| + | |
| - | <div>
| + | |
| - | The growth in the number of cells is described with a typical logistic function,
| + | |
| - | whose parameters have been previously characterized.
| + | |
| - | </div>
| + | |
| | | | |
| | | | |
 "
"