|
JULY: WEEK 2
July, 4th
Digestions of previously purified plasmids were performed for ligations:
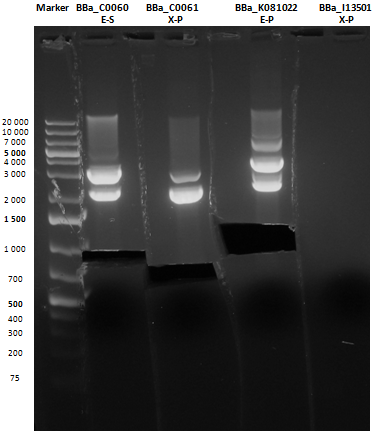
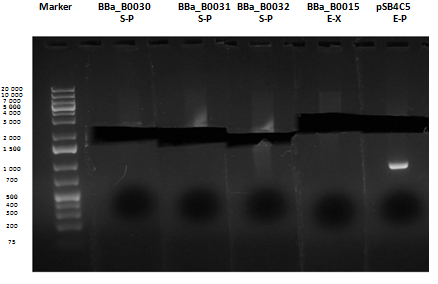
Reactions were incubated at 37°C for three hours while a small-size and a medium-size agarose gel were prepared according to protocols. In the afternoon gel electrophoresis was performed. As shown, in figure all clones were positive (apart from BBa_I13501 run where we inexplicably didn't see any band), so we cut and purified the bands of interest. Cells harbouring BBa_I13501 were again inoculated in 5 ml LB + Amp. After gel extraction, digested DNA was quantified:
Then ligations were performed in a final volume of 10 μl:
Ligations were incubated ON at 16°C. July, 5th
T4 Ligase was inactivated, heating ligations at 65°C for 10 minutes; ligations were stored at -20°C. Plasmid purification was done again for BBa_I13501 and purified DNA was quantified:
BBa_I13501 was then digested with restriction endonucleases:
According to protocols the reaction was incubated at 37°C for 3 hours. Digested DNA was gel-extracted:
Further ligations were prepared and incubated ON at 16°C:
July, 6th
BBa_C0261 was resuspended from iGEM 2011 kit distribution, Plate 1, well 14C in 15 μl of ddH2O; BBa_C0261, E1, E2, E3, E4, E5, E6, E7, and E8 were transformed in 100 μl of TOP10 competent cells according to protocols. Plates were incubated ON at 37°C. July, 7th
All plates showed a lot of colonies, except for E-8 (5 colonies); two colonies for E1, E2, E3, E4, E5, E6, E7 plates and three colonies for E8 plate were picked and inoculated in 10 μl LB for inoculum and screening PCR.
24 μl were aliquoted in each tube, together with 1 μl of each liquid culture. PCR cycles parameters were set as follows:
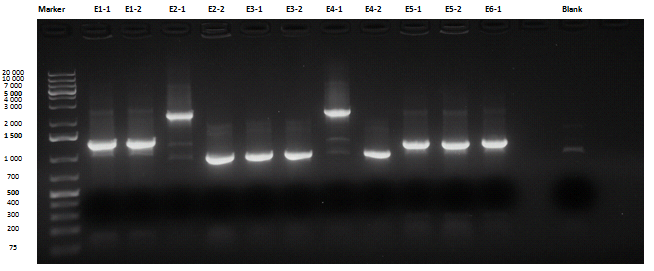
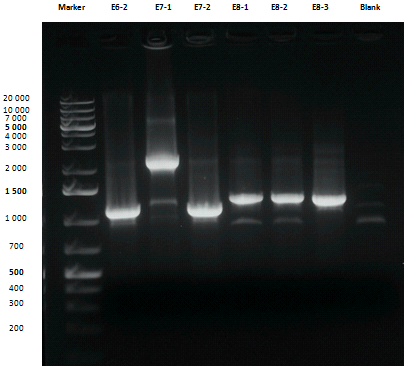
Liquid cultures were then inoculated in 800 μl LB with the proper antibiotic. A small size and a medium size agarose gel were prepared. After the PCR, gel run was carried out on the samples:
Except for E2-1, E4-1 and E7-1, other band lengths were correct; 750 μl of E1-2, E2-2, E3-1, E4-2, E5-2, E6-1, E7-2, E8-3 and BBa_C0261 liquid cultures were used to prepare glycerol stocks while the remaining 50 μl were refilled to 5 ml for plasmid purification and incubated at 37°C 220 rpm. BBa_R0040, BBa_C0261 and BBa_I13521 were also inoculated. July, 8thCultures were grown till saturation; plasmid purification was carried out:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Team:UNIPV-Pavia/Calendar/July/settimana2
From 2011.igem.org
(Difference between revisions)
| (43 intermediate revisions not shown) | |||
| Line 20: | Line 20: | ||
<center> | <center> | ||
| - | <table | + | <table class="data"> |
<tr> | <tr> | ||
| - | <td><b>Plasmid</b></td> | + | <td class="row"><b>Plasmid</b></td> |
| - | <td><b>Kind</b></td> | + | <td class="row"><b>Kind</b></td> |
| - | <td><b> | + | <td class="row"><b>DNA (μl)</b></td> |
| - | <td><b>H<small><sub>2</sub></small>O (μl)</b></td> | + | <td class="row"><b>H<small><sub>2</sub></small>O (μl)</b></td> |
| - | <td><b>Enzyme 1 (μl)</b></td> | + | <td class="row"><b>Enzyme 1 (μl)</b></td> |
| - | <td><b>Enzyme 2 (μl)</b></td> | + | <td class="row"><b>Enzyme 2 (μl)</b></td> |
| - | <td><b>Buffer H (μl)</b></td> | + | <td class="row"><b>Buffer H (μl)</b></td> |
| - | <td><b>Final Volume(μl)</b></td> | + | <td class="row"><b>Final Volume (μl)</b></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0060">BBa_C0060</a></td> |
| - | <td>Insert</td> | + | <td class="row">Insert</td> |
| - | <td>16.5</td> | + | <td class="row">16.5</td> |
| - | <td>4</td> | + | <td class="row">4</td> |
| - | <td>1 EcoRI</td> | + | <td class="row">1 EcoRI</td> |
| - | <td>1 SpeI</td> | + | <td class="row">1 SpeI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0061">BBa_C0061</a></td> |
| - | <td>Insert</td> | + | <td class="row">Insert</td> |
| - | <td>13.2</td> | + | <td class="row">13.2</td> |
| - | <td>7.3</td> | + | <td class="row">7.3</td> |
| - | <td>1 XbaI</td> | + | <td class="row">1 XbaI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K081022">BBa_K081022</a></td> |
| - | <td>Insert</td> | + | <td class="row">Insert</td> |
| - | <td>15.7</td> | + | <td class="row">15.7</td> |
| - | <td>4.8</td> | + | <td class="row">4.8</td> |
| - | <td>1 EcoRI</td> | + | <td class="row">1 EcoRI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0030">BBa_B0030</a></td> |
| - | <td>Vector</td> | + | <td class="row">Vector</td> |
| - | <td>13.2</td> | + | <td class="row">13.2</td> |
| - | <td>7.3</td> | + | <td class="row">7.3</td> |
| - | <td>1 SpeI</td> | + | <td class="row">1 SpeI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0031">BBa_B0031</a></td> |
| - | <td>Vector</td> | + | <td class="row">Vector</td> |
| - | <td>12.4</td> | + | <td class="row">12.4</td> |
| - | <td>8.1</td> | + | <td class="row">8.1</td> |
| - | <td>1 SpeI</td> | + | <td class="row">1 SpeI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0032">BBa_B0032</a></td> |
| - | <td>Vector</td> | + | <td class="row">Vector</td> |
| - | <td>9.5</td> | + | <td class="row">9.5</td> |
| - | <td>11</td> | + | <td class="row">11</td> |
| - | <td>1 SpeI</td> | + | <td class="row">1 SpeI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0015">BBa_B0015</a></td> |
| - | <td>Vector</td> | + | <td class="row">Vector</td> |
| - | <td>7.9</td> | + | <td class="row">7.9</td> |
| - | <td>12.6</td> | + | <td class="row">12.6</td> |
| - | <td>1 EcoRI</td> | + | <td class="row">1 EcoRI</td> |
| - | <td>1 XbaI</td> | + | <td class="row">1 XbaI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:pSB4C5">pSB4C5</a></td> |
| - | <td>Vector</td> | + | <td class="row">Vector</td> |
| - | <td>3</td> | + | <td class="row">3</td> |
| - | <td>17.5</td> | + | <td class="row">17.5</td> |
| - | <td>1 EcoRI</td> | + | <td class="row">1 EcoRI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td></ | + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a></td> |
| - | <td>Insert</td> | + | <td class="row">Insert</td> |
| - | <td>7.2</td> | + | <td class="row">7.2</td> |
| - | <td>13.3</td> | + | <td class="row">13.3</td> |
| - | <td>1 XbaI</td> | + | <td class="row">1 XbaI</td> |
| - | <td>1 PstI</td> | + | <td class="row">1 PstI</td> |
| - | <td>2.5</td> | + | <td class="row">2.5</td> |
| - | <td>25</td> | + | <td class="row">25</td> |
</tr> | </tr> | ||
| Line 142: | Line 142: | ||
<p> | <p> | ||
| - | + | Reactions were incubated at 37°C for three hours while a small-size and a medium-size agarose gel were prepared according to protocols. | |
</p> | </p> | ||
| Line 148: | Line 148: | ||
In the afternoon gel electrophoresis was performed. | In the afternoon gel electrophoresis was performed. | ||
</p> | </p> | ||
| + | |||
| + | <div class="center"><div class="thumb tnone"><div class="thumbinner" style="width: 377px;"><a href="/File:UNIPV_04_07_SSgel.png" class="image"><img alt="" src="/wiki/images/4/47/UNIPV_04_07_SSgel.png" class="thumbimage" height="432" width="375"></a> <div class="thumbcaption">Small size gel</div></div></div></div> | ||
| + | <div class="center"><div class="thumb tnone"><div class="thumbinner" style="width: 400px;"><a href="/File:UNIPV_04_07_MSgel.png" class="image"><img alt="" src="/wiki/images/5/59/UNIPV_04_07_MSgel.png" class="thumbimage" height="80%" width="80%"></a> <div class="thumbcaption">Medium size gel</div></div></div></div> | ||
| + | |||
| + | |||
| + | |||
| + | <p> | ||
| + | As shown, in figure all clones were positive (apart from <a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> run where we inexplicably didn't see any band), so we cut and purified the bands of interest. Cells harbouring <a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> were again inoculated in 5 ml LB + Amp. | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | After gel extraction, digested DNA was quantified: | ||
| + | </p> | ||
| + | |||
| + | |||
<center> | <center> | ||
| - | + | <table class="data"> | |
<tr> | <tr> | ||
| - | + | <td class="row"><b>Part</b></td> | |
| - | + | <td class="row"><b>DNA (ng/μl)</b></td> | |
| - | + | </tr> | |
| - | </table> | + | |
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0060">BBa_C0060</a> (E-S)</td> | ||
| + | <td class="row">3.9</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0061">BBa_C0061</a> (X-P)</td> | ||
| + | <td class="row">4.1</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K081022">BBa_K081022</a> (E-P)</td> | ||
| + | <td class="row">4.4</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0030">BBa_B0030</a> (S-P)</td> | ||
| + | <td class="row">10.3</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0031">BBa_B0031</a> (S-P)</td> | ||
| + | <td class="row">11.8</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0032">BBa_B0032</a> (S-P)</td> | ||
| + | <td class="row">10.1</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0015">BBa_B0015</a> (E-X)</td> | ||
| + | <td class="row">12</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:pSB4C5">pSB4C5</a> (E-P)</td> | ||
| + | <td class="row">10.7</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
</center> | </center> | ||
| - | + | <p> | |
| + | Then ligations were performed in a final volume of 10 μl: | ||
| + | </p> | ||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>Ligation Name</b></td> | ||
| + | <td class="row"><b>Vector</b></td> | ||
| + | <td class="row"><b>Vector volume (μl)</b></td> | ||
| + | <td class="row"><b>Insert</b></td> | ||
| + | <td class="row"><b>Insert volume (μl)</b></td> | ||
| + | <td class="row"><b>Buffer (μl)</b></td> | ||
| + | <td class="row"><b>T4 Ligase (μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E1</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0015">BBa_B0015</a> (E-X)</td> | ||
| + | <td class="row">1.5</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0060">BBa_C0060</a> (E-S)</td> | ||
| + | <td class="row">6.5</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E2</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0030">BBa_B0030</a> (S-P)</td> | ||
| + | <td class="row">1.5</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0061">BBa_C0061</a> (X-P)</td> | ||
| + | <td class="row">6.5</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E3</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0031">BBa_B0031</a> (S-P)</td> | ||
| + | <td class="row">1.5</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0061">BBa_C0061</a> (X-P)</td> | ||
| + | <td class="row">6.5</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E4</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0032">BBa_B0032</a> (S-P)</td> | ||
| + | <td class="row">1.5</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0061">BBa_C0061</a> (X-P)</td> | ||
| + | <td class="row">6.5</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E8</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:pSB4C5">pSB4C5</a> (E-P)</td> | ||
| + | <td class="row">1.5</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K081022">BBa_K081022</a> (E-P)</td> | ||
| + | <td class="row">6.5</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <p> | ||
| + | Ligations were incubated ON at 16°C. | ||
| + | </p> | ||
<div align="right"><small><a href="#indice" title="">^top</a></small></div> | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | ||
| + | |||
| + | </p> | ||
| + | <a name="July.2C_5th"></a><h2> <span class="mw-headline">July, 5th</span></h2> | ||
| + | <p> | ||
| + | |||
| + | <p> | ||
| + | T4 Ligase was inactivated, heating ligations at 65°C for 10 minutes; ligations were stored at -20°C. Plasmid purification was done again for <a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> and purified DNA was quantified: | ||
| + | </p> | ||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>Plasmid</b></td> | ||
| + | <td class="row"><b>DNA (ng/μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a></td> | ||
| + | <td class="row">97.2</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <p> | ||
| + | <a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> was then digested with restriction endonucleases: | ||
| + | </p> | ||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>Plasmid</b></td> | ||
| + | <td class="row"><b>Kind</b></td> | ||
| + | <td class="row"><b>DNA (μl)</b></td> | ||
| + | <td class="row"><b>H<small><sub>2</sub></small>O (μl)</b></td> | ||
| + | <td class="row"><b>Enzyme 1 (μl)</b></td> | ||
| + | <td class="row"><b>Enzyme 2 (μl)</b></td> | ||
| + | <td class="row"><b>Buffer H (μl)</b></td> | ||
| + | <td class="row"><b>Final Volume (μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a></td> | ||
| + | <td class="row">Insert</td> | ||
| + | <td class="row">10.5</td> | ||
| + | <td class="row">10</td> | ||
| + | <td class="row">1 XbaI</td> | ||
| + | <td class="row">1 PstI</td> | ||
| + | <td class="row">2.5</td> | ||
| + | <td class="row">25</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <p> | ||
| + | According to protocols the reaction was incubated at 37°C for 3 hours.<br> | ||
| + | 60 ml of 80% glycerol were prepared mixing 48 ml of 100% glycerol with 12 ml of ddH<small><sub>2</small></sub>O.<br> | ||
| + | Gel electrophoresis was done for <a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> digestion: | ||
| + | </p> | ||
| + | |||
| + | <div class="center"><div class="thumb tnone"><div class="thumbinner" style="width: 171px;"><a href="/File:UNIPV_05_07_SSgel.png" class="image"><img alt="" src="/wiki/images/c/c3/UNIPV_05_07_SSgel.png" class="thumbimage" height="451" width="169"></a> <div class="thumbcaption">Small size gel</div></div></div></div> | ||
| + | |||
| + | |||
| + | |||
| + | <p> | ||
| + | Digested DNA was gel-extracted: | ||
| + | </p> | ||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>Part</b></td> | ||
| + | <td class="row"><b>DNA (ng/μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> (X-P)</td> | ||
| + | <td class="row">2.6</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <p> | ||
| + | Further ligations were prepared and incubated ON at 16°C: | ||
| + | </p> | ||
| + | |||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>Ligation Name</b></td> | ||
| + | <td class="row"><b>Vector</b></td> | ||
| + | <td class="row"><b>Vector volume (μl)</b></td> | ||
| + | <td class="row"><b>Insert</b></td> | ||
| + | <td class="row"><b>Insert volume (μl)</b></td> | ||
| + | <td class="row"><b>Buffer (μl)</b></td> | ||
| + | <td class="row"><b>T4 Ligase (μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E5</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0030">BBa_B0030</a> (S-P)</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> (X-P)</td> | ||
| + | <td class="row">7</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E6</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0031">BBa_B0031</a> (S-P)</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> (X-P)</td> | ||
| + | <td class="row">7</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><b>E7</b></td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0032">BBa_B0032</a> (S-P)</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13501">BBa_I13501</a> (X-P)</td> | ||
| + | <td class="row">7</td> | ||
| + | <td class="row">1</td> | ||
| + | <td class="row">1</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <a name="July.2C_6th"></a><h2> <span class="mw-headline">July, 6th</span></h2> | ||
| + | <p> | ||
| + | <a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0261">BBa_C0261</a> was resuspended from iGEM 2011 kit distribution, Plate 1, well 14C in 15 μl of ddH<small><sub>2</sub></small>O; <a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0261">BBa_C0261</a>, E1, E2, E3, E4, E5, E6, E7, and E8 were transformed in 100 μl of TOP10 competent cells according to protocols. Plates were incubated ON at 37°C.<br> | ||
| + | 500 ml of LB without antibiotic were prepared. | ||
| + | </p> | ||
| + | |||
| + | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | ||
| + | |||
| + | <a name="July.2C_7th"></a><h2> <span class="mw-headline">July, 7th</span></h2> | ||
| + | <p> | ||
| + | All plates showed a lot of colonies, except for E-8 (5 colonies); two colonies for E1, E2, E3, E4, E5, E6, E7 plates and three colonies for E8 plate were picked and inoculated in 10 μl LB for inoculum and screening PCR.<br> | ||
| + | A 20x mix was prepared for PCR reaction: | ||
| + | </p> | ||
| + | |||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>H<small><sub>2</sub></small>O (μl)</b></td> | ||
| + | <td class="row"><b>Buffer 10x (μl)</b></td> | ||
| + | <td class="row"><b>MgCl<small><sub>2</sub></small> (μl)</b></td> | ||
| + | <td class="row"><b>VF2 (<a href="http://partsregistry.org/wiki/index.php/Part:BBa_G00100">BBa_G00100</a>) μl</b></td> | ||
| + | <td class="row"><b>VR (<a href="http://partsregistry.org/wiki/index.php/Part:BBa_G00101">BBa_G00101</a>) μl</b></td> | ||
| + | <td class="row"><b>dNTPs (μl)</b></td> | ||
| + | <td class="row"><b>Taq polymerase (μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row">360</td> | ||
| + | <td class="row">50</td> | ||
| + | <td class="row">20</td> | ||
| + | <td class="row">10</td> | ||
| + | <td class="row">10</td> | ||
| + | <td class="row">10</td> | ||
| + | <td class="row">20</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <p> | ||
| + | 24 μl were aliquoted in each tube, together with 1 μl of each liquid culture. PCR cycles parameters were set as follows: | ||
| + | <ul type="circle"> | ||
| + | <li> 94°C 30 seconds (denaturing) | ||
| + | <li> 60°C 1 minute (annealing) | ||
| + | <li> 72°C 1 minute and 20 seconds (elongation) | ||
| + | </ul> | ||
| + | 35 cycles were performed.<br> | ||
| + | Liquid cultures were then inoculated in 800 μl LB with the proper antibiotic.<br> | ||
| + | A small size and a medium size agarose gel were prepared. | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | After the PCR, gel run was carried out on the samples: | ||
| + | </p> | ||
| + | |||
| + | <div class="center"><div class="thumb tnone"><div class="thumbinner" style="width: 400px;"><a href="/File:UNIPV_07_07_MSgel.png" class="image"><img alt="" src="/wiki/images/1/12/UNIPV_07_07_MSgel.png" class="thumbimage" height="80%" width="80%"></a> <div class="thumbcaption">Medium size gel</div></div></div></div> | ||
| + | <div class="center"><div class="thumb tnone"><div class="thumbinner" style="width: 400px;"><a href="/File:UNIPV_07_07_SSgel.png" class="image"><img alt="" src="/wiki/images/7/73/UNIPV_07_07_SSgel.png" class="thumbimage" height="80%" width="80%"></a> <div class="thumbcaption">Small size gel</div></div></div></div> | ||
| + | |||
| + | <p> | ||
| + | Except for E2-1, E4-1 and E7-1, other band lengths were correct; 750 μl of E1-2, E2-2, E3-1, E4-2, E5-2, E6-1, E7-2, E8-3 and <a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0261">BBa_C0261</a> liquid cultures were used to prepare glycerol stocks while the remaining 50 μl were refilled to 5 ml for plasmid purification and incubated at 37°C 220 rpm. <a href="http://partsregistry.org/wiki/index.php/Part:BBa_R0040">BBa_R0040</a>, <a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0261">BBa_C0261</a> and <a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13521">BBa_I13521</a> were also inoculated.<br> | ||
| + | In the late afternoon the team met to talk about the wet-lab activity, the abstract and the bio-safety section. | ||
| + | </p> | ||
| + | |||
| + | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | ||
| + | |||
| + | <a name="July.2C_8th"></a><h2> <span class="mw-headline">July, 8th</span></h2> | ||
| + | |||
| + | <p> | ||
| + | Cultures were grown till saturation; plasmid purification was carried out: | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | <center> | ||
| + | <table class="data"> | ||
| + | <tr> | ||
| + | <td class="row"><b>Plasmid</b></td> | ||
| + | <td class="row"><b>DNA (ng/μl)</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row">E1-2</td> | ||
| + | <td class="row">64.1</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row">E2-2</td> | ||
| + | <td class="row">56.2</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row">E3-1</td> | ||
| + | <td class="row">53.5</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | <tr> | ||
| + | <td class="row">E4-2</td> | ||
| + | <td class="row">67.2</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row">E5-2</td> | ||
| + | <td class="row">73.5</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row">E6-1</td> | ||
| + | <td class="row">92.2</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row">E7-2</td> | ||
| + | <td class="row">67.6</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row">E8-3</td> | ||
| + | <td class="row">25.3</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_R0040">BBa_R0040</a></td> | ||
| + | <td class="row">48.1</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_C0261">BBa_C0261</a></td> | ||
| + | <td class="row">61.2</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td class="row"><a href="http://partsregistry.org/wiki/index.php/Part:BBa_I13521">BBa_I13521</a></td> | ||
| + | <td class="row">79.3</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </center> | ||
| + | |||
| + | <div align="right"><small><a href="#indice" title="">^top</a></small></div> | ||
| + | |||
<table border="0" width="100%" class="menu"> | <table border="0" width="100%" class="menu"> | ||
<tr> | <tr> | ||
| - | <td align="left"><a href="/Team:UNIPV-Pavia/Calendar/ | + | <td align="left"><a href="/Team:UNIPV-Pavia/Calendar/July/settimana1" title="Team:UNIPV-Pavia/Calendar/JuLY/settimana1"> Previous week</a></td> |
| - | <td align="right"><a href="/Team:UNIPV-Pavia/Calendar/July/ | + | <td align="right"><a href="/Team:UNIPV-Pavia/Calendar/July/settimana3" title="Team:UNIPV-Pavia/Calendar/July/settimana3"> Next week</a></td> |
</tr> | </tr> | ||
| Line 172: | Line 583: | ||
</html> | </html> | ||
| - | {{ | + | |
| + | |||
| + | |||
| + | {{endcalendar}} | ||
Latest revision as of 14:26, 17 August 2011
 "
"