Team:Arizona State/Notebook/PCRLog
From 2011.igem.org
(Difference between revisions)
(Created page with "{{:Team:Arizona State/Templates/main|title=PCR Log for E. Coli Cas Genes|content= __NOTOC__ ==Overview== <p>This logbook is a record of the majority of our attempts to PCR ampli...") |
Ethan ward (Talk | contribs) |
||
| (15 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
===Notes=== | ===Notes=== | ||
<p>Suggested annealing temperatures are based on [http://www.neb.com/nebecomm/tech_reference/TmCalc/Default.asp NEB Tm Calculator], as called for in the [http://www.neb.com/nebecomm/tech_reference/polymerases/phusion_high.asp?&utm_source=Google&utm_medium=CPC&utm_term=+phusion&utm_campaign=Phusion NEB Phusion DNA Polymerase] protocol.</p> | <p>Suggested annealing temperatures are based on [http://www.neb.com/nebecomm/tech_reference/TmCalc/Default.asp NEB Tm Calculator], as called for in the [http://www.neb.com/nebecomm/tech_reference/polymerases/phusion_high.asp?&utm_source=Google&utm_medium=CPC&utm_term=+phusion&utm_campaign=Phusion NEB Phusion DNA Polymerase] protocol.</p> | ||
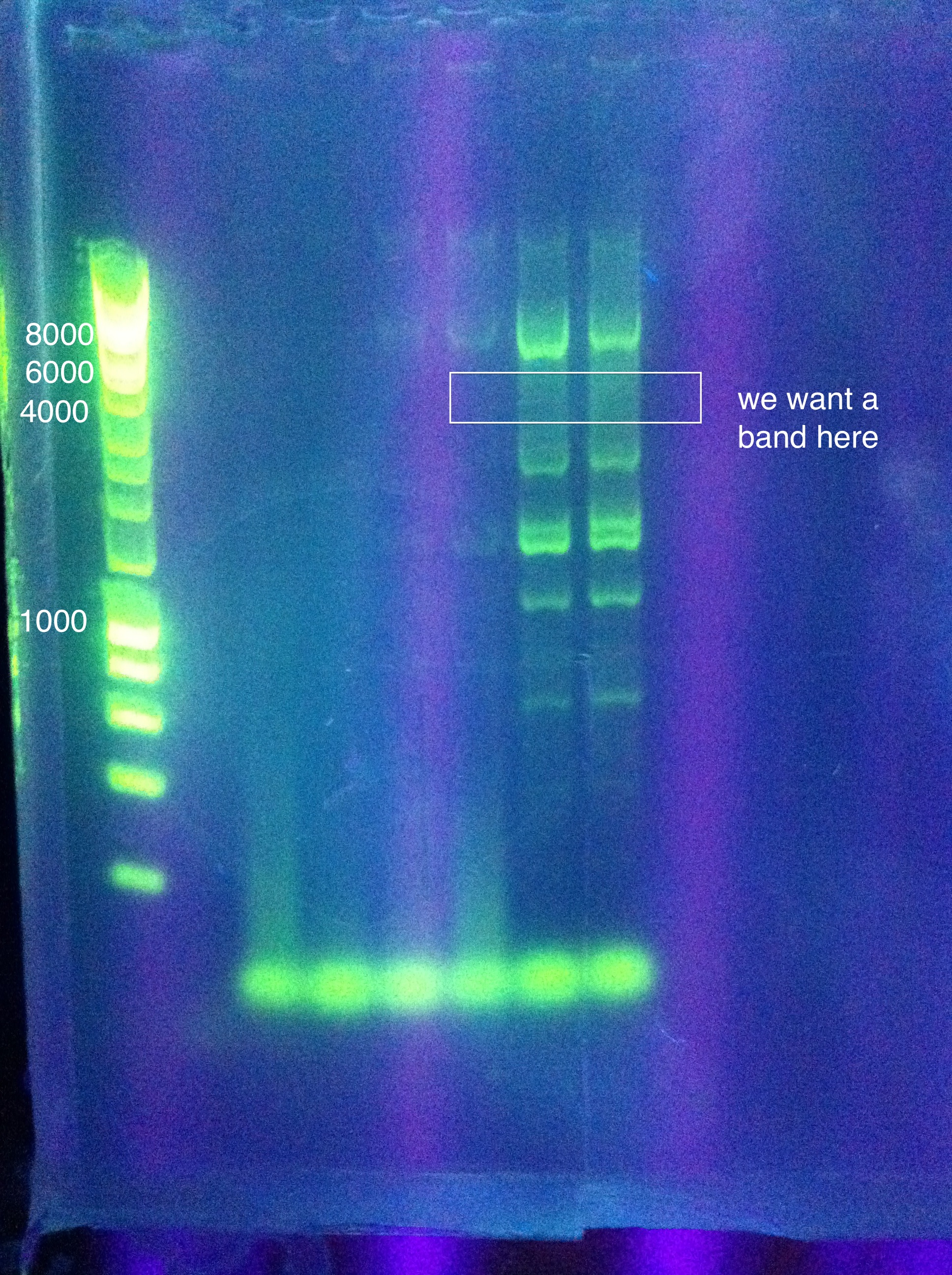
| + | <p>Desired band for CasABCDE at ~4300bp</p> | ||
| + | <p>Desired band for Cas3 at ~2667bp</p> | ||
==Primer Round 1== | ==Primer Round 1== | ||
===July 17, 2011=== | ===July 17, 2011=== | ||
| - | <b>CasABCDE</b>: Touchdown PCR, Start Temp 70, -0.2 / cycle, Final Temp 64 | + | <b>CasABCDE</b>: Touchdown PCR, Start Temp 70, -0.2 / cycle, Final Temp 64 |
| - | <br><b>Cas3</b>: Touchdown PCR, Start Temp 63, -0.2 / cycle, Final Temp 57 | + | <br><b>Cas3</b>: Touchdown PCR, Start Temp 63, -0.2 / cycle, Final Temp 57 |
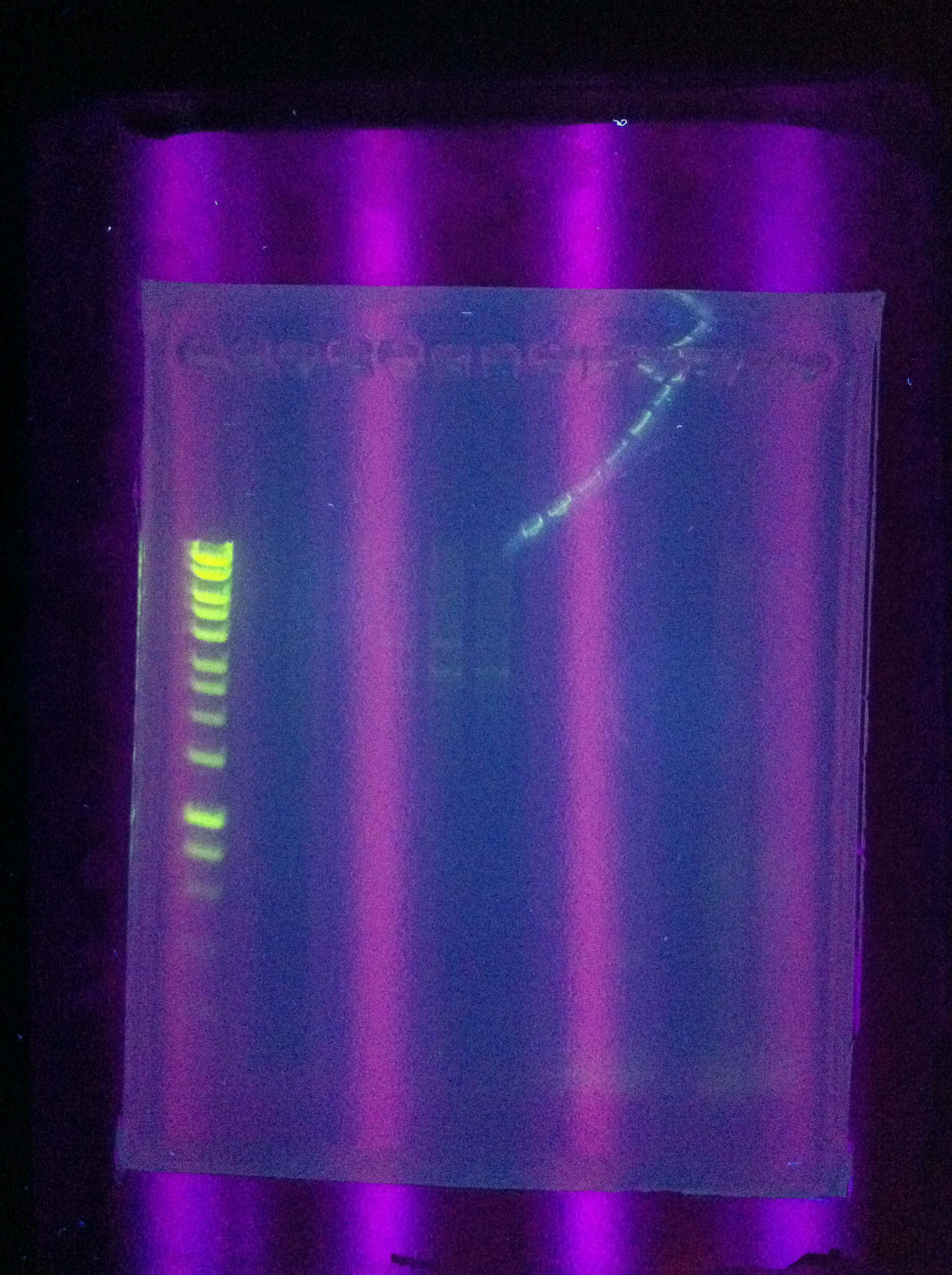
<p>Gel Results:</p> | <p>Gel Results:</p> | ||
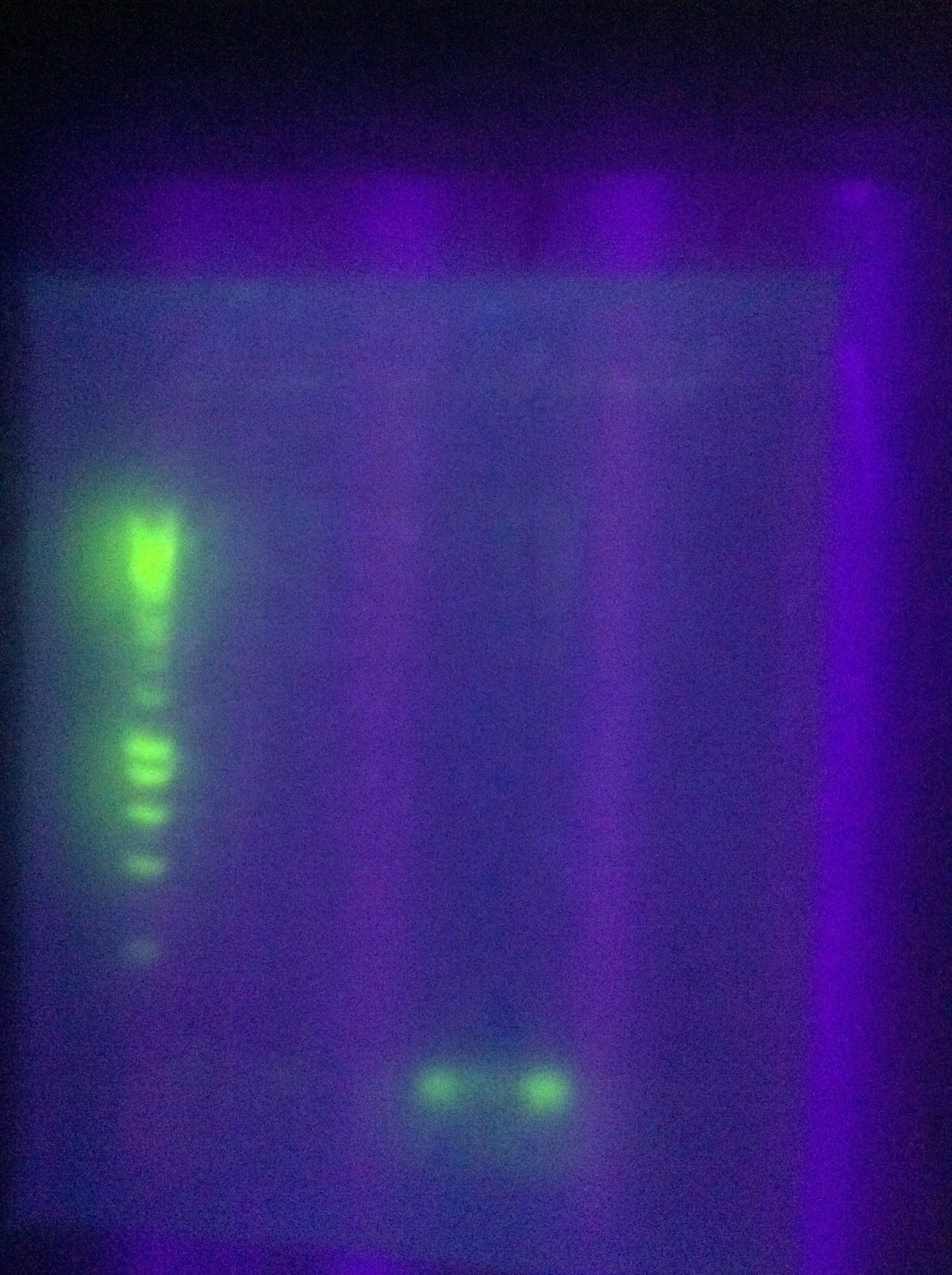
| - | [[Image:]] | + | [[Image:ASU_717_cas_gel.jpg|300px]] |
| + | <br>CasABCDE: Very faint bands near target length | ||
| + | <br>Cas3: No bands | ||
| + | ===July 18, 2011=== | ||
| + | <b>CasABCDE</b>: Touchdown PCR, Start Temp 65, -0.2 / cycle, Final Temp 59 | ||
| + | <p>Gel Results:</p> | ||
| + | CasABCDE: No bands | ||
| + | |||
| + | ===July 19, 2011=== | ||
| + | <b>CasABCDE</b>: Touchdown PCR, Start Temp 70, -0.2 / cycle, Final Temp 63 | ||
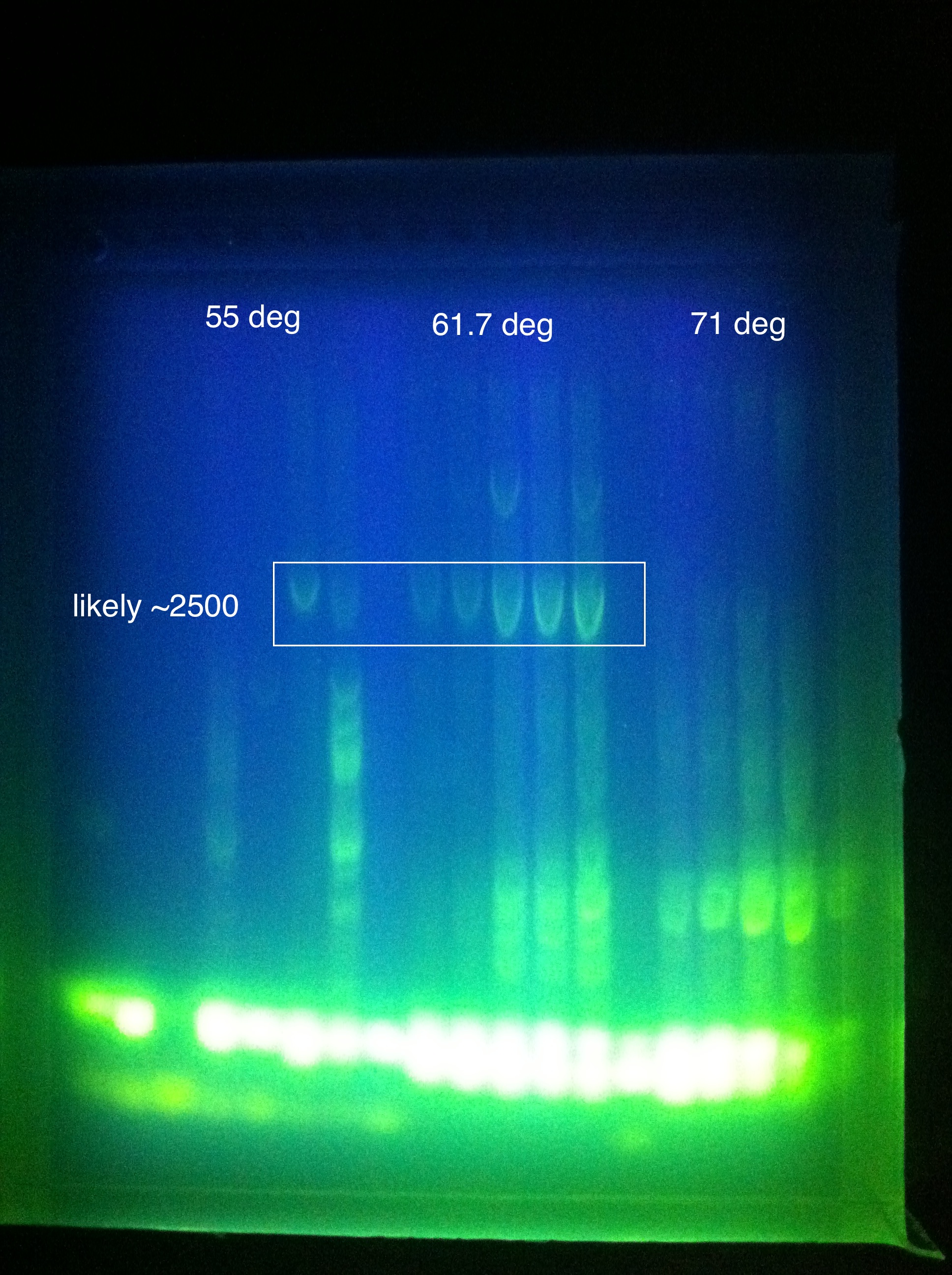
| + | <br><b>Cas3</b>: Temp gradient with 3 rows from 72 to 55 (L to R: 55, 61.7, 71) | ||
| + | <br>Gel Results: | ||
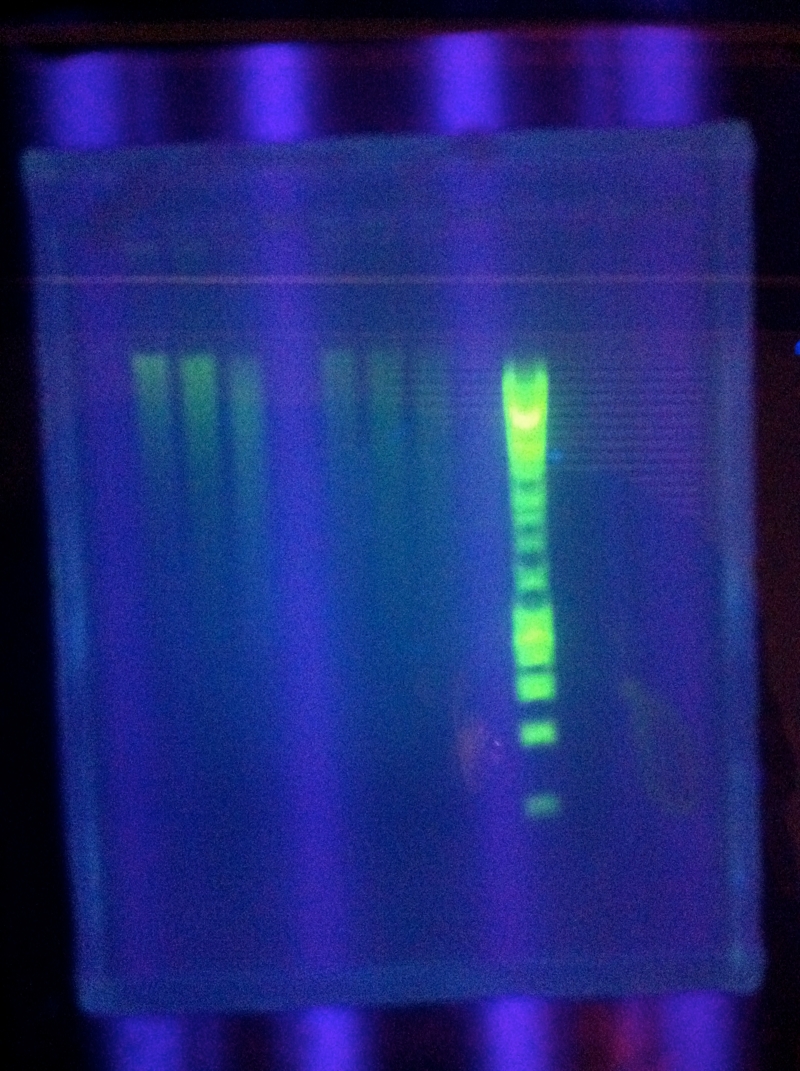
| + | <br>[[Image:ASU_719_casABCDE.jpg|300px]] | ||
| + | <br>CasABCDE: Clearer bands around target length, primer dimers | ||
| + | <br>[[Image:ASU_719_cas3.jpg|300px]] | ||
| + | <br>Cas3: Middle temperature was optimal, clear bands are visible (likely correct length, failed to run a hyperladder), primer dimers | ||
| + | |||
| + | ===July 20, 2011=== | ||
| + | <b>CasABCDE</b>: Touchdown PCR, Start Temp 71, -0.2 / cycle, Final Temp 64 | ||
| + | <br><b>Cas3</b>: Gradient using tighter range around best temp from yesterday (65.6, 63.1, 61.2, 58.5) | ||
| + | <p>Gel Results:</p> | ||
| + | [[Image:ASU_720_CasABCDE.jpg|300px]] | ||
| + | <br>CasABCDE: Nonspecific, nothing usable, no bands in target range, dimerization | ||
| + | <br>[[Image:ASU_720_Cas3.jpg|300px]] | ||
| + | <br>Cas3: Small band at correct location, primer dimers | ||
| + | |||
| + | ===July 24, 2011=== | ||
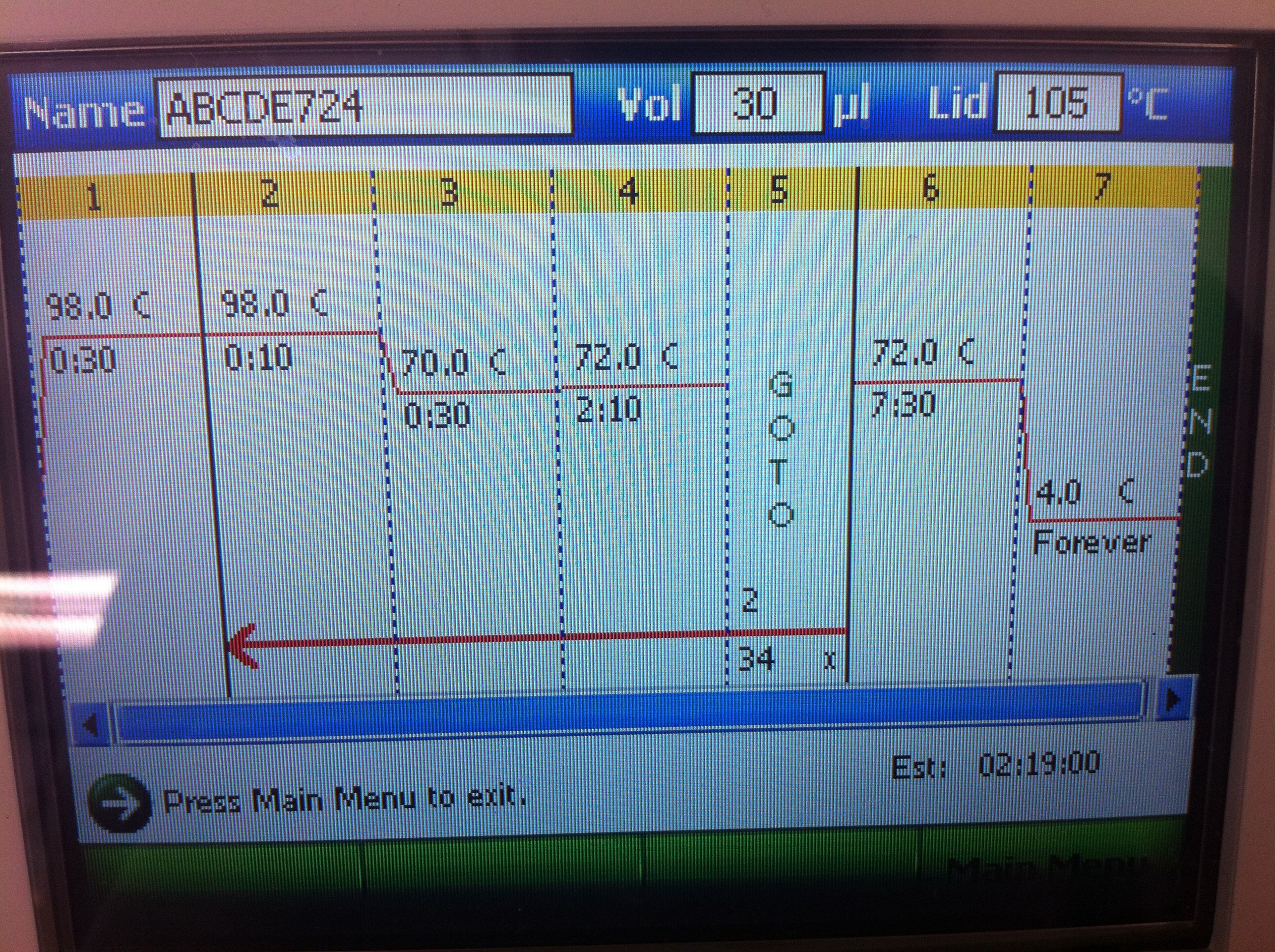
| + | <p>PCR of CasABCDE, Cas3 with new settings; Preparation for extraction, "PCRception" (PCRing the PCR results); Included an elongation step in each cycle, which was not done for the previous runs; Settings stored in PCR machine as ABCDE724, CAS3724 | ||
| + | </p> | ||
| + | <b>CasABCDE Settings and Results</b>: | ||
| + | <br>[[Image:ASU_725_Settings_ABCDE.jpeg|300px]] | ||
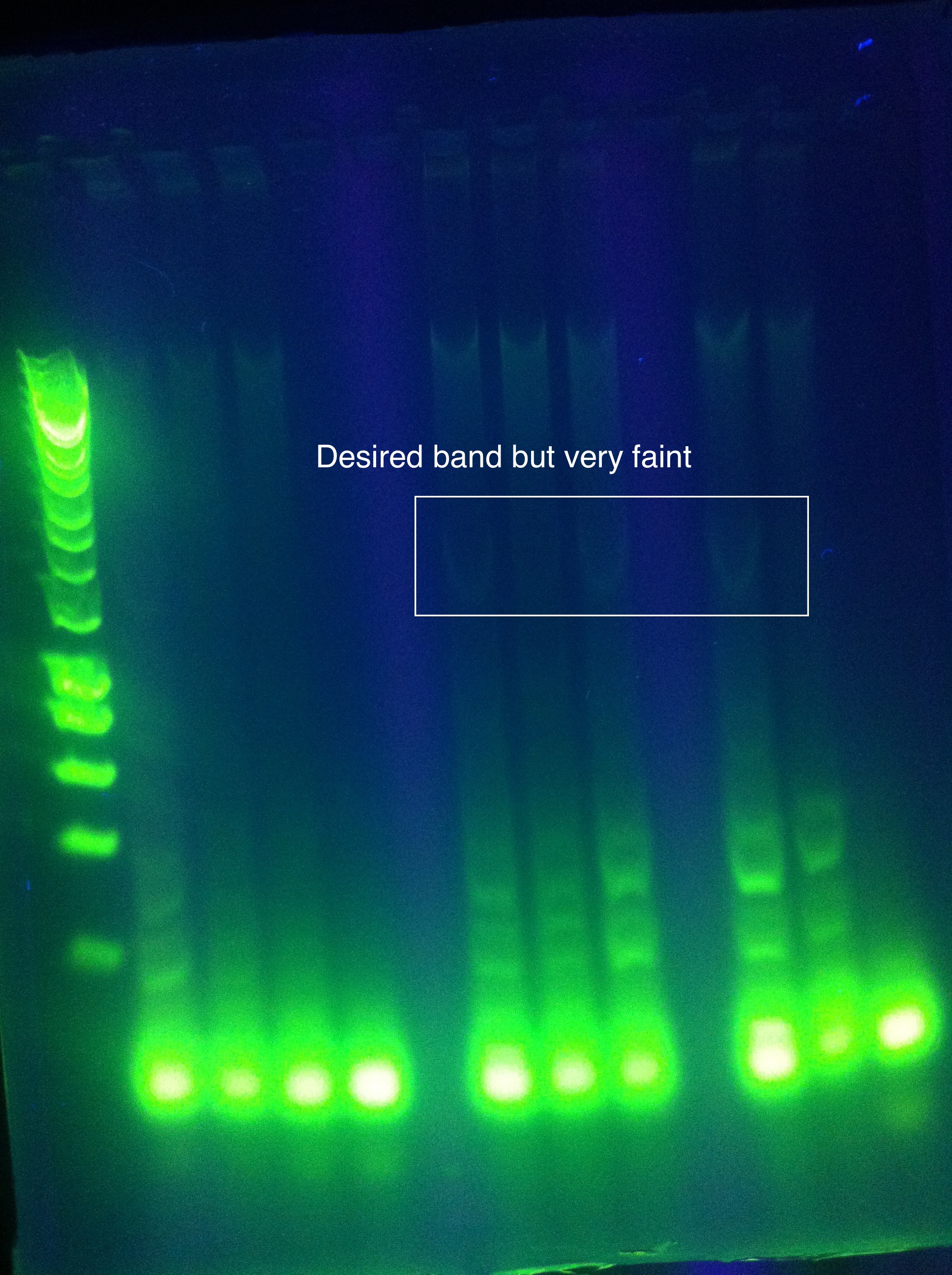
| + | <br><br>[[Image:ASU_725_gel_abcde.jpeg|300px]] | ||
| + | <br>Nonspecific results, perhaps a hint of the correct bands in the second to last well | ||
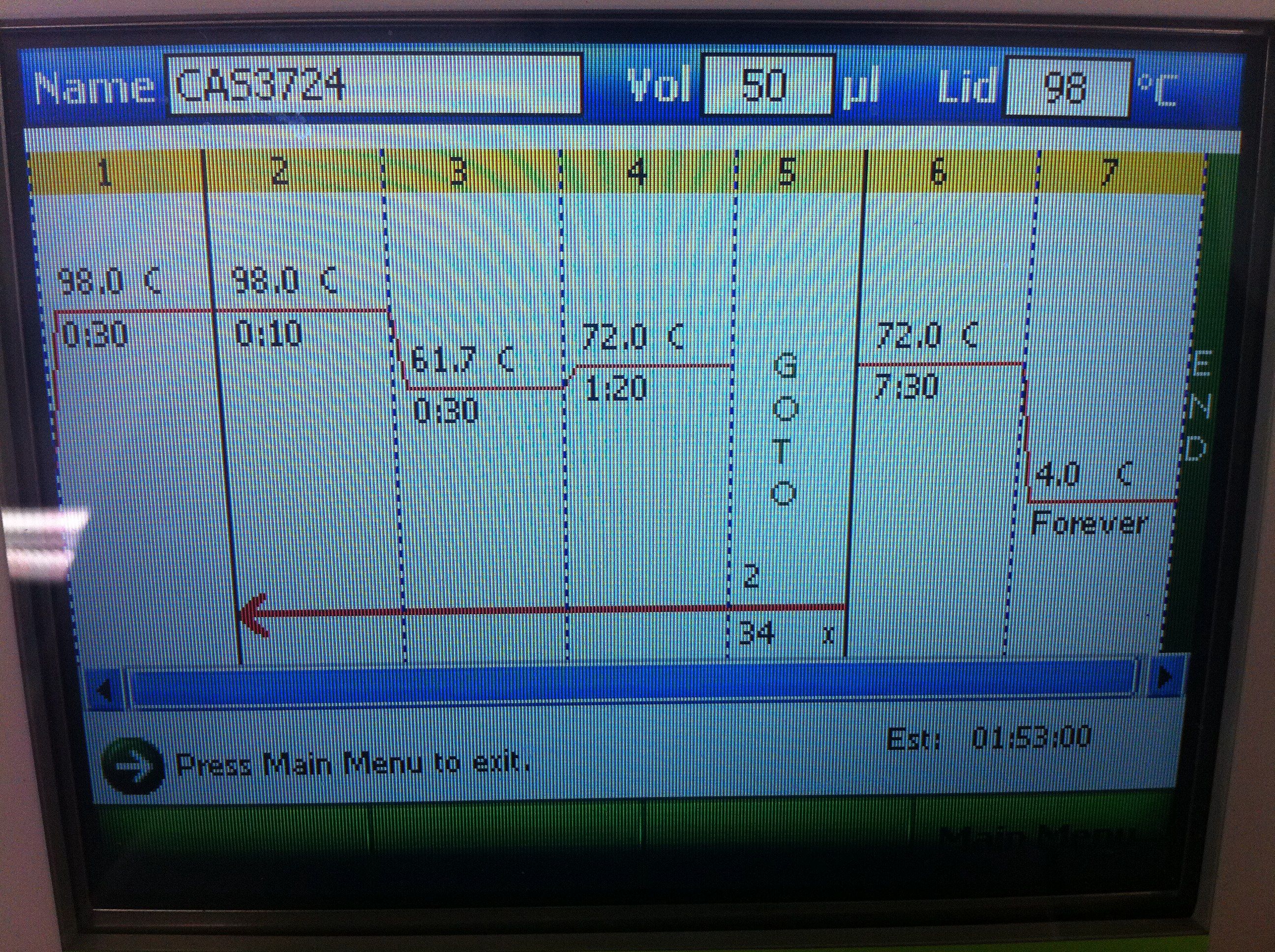
| + | <br><b>Cas3 Settings and Results</b>: | ||
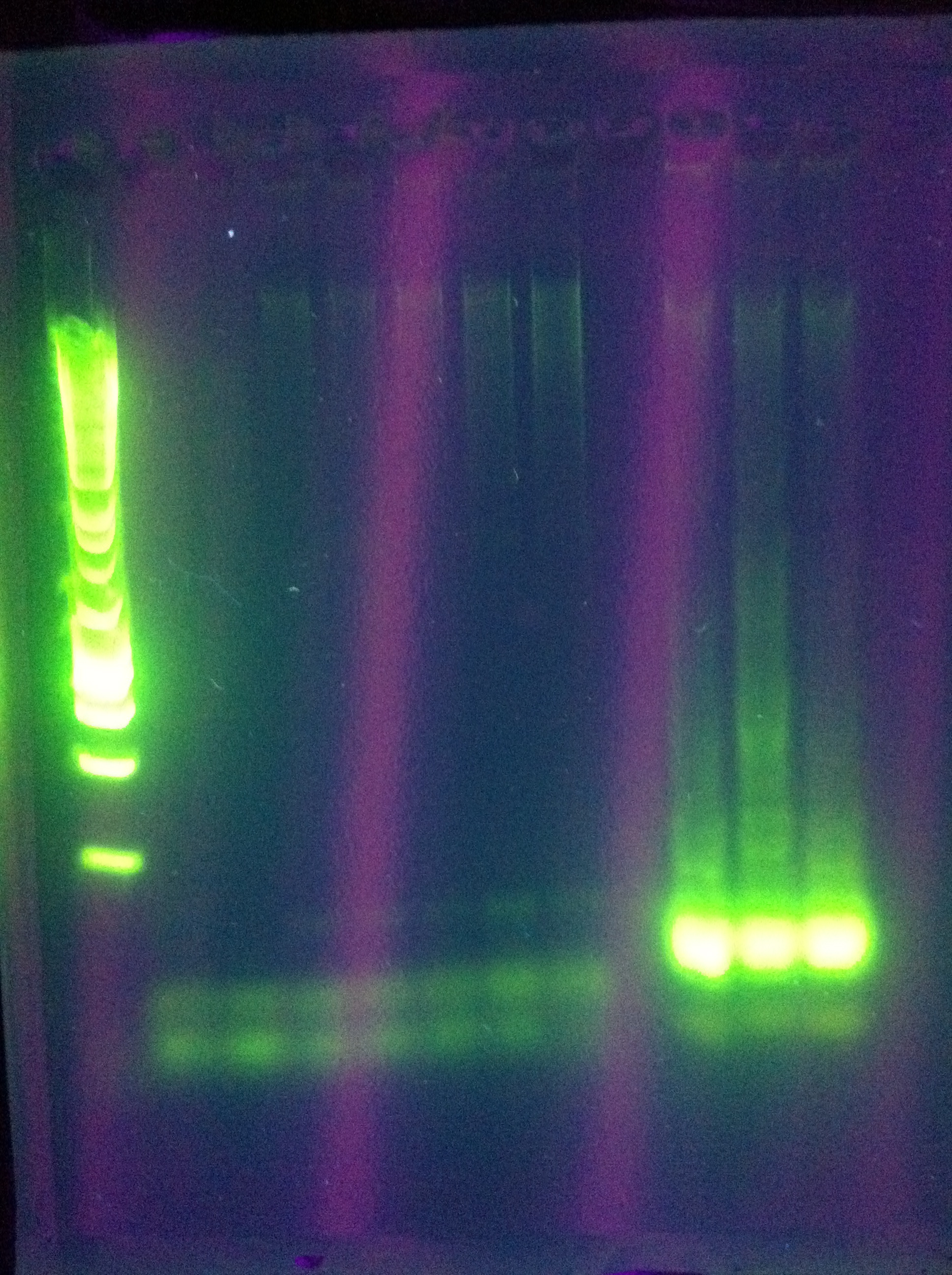
| + | <br>[[Image:ASU_725_Settings_Cas3.jpeg|300px]] | ||
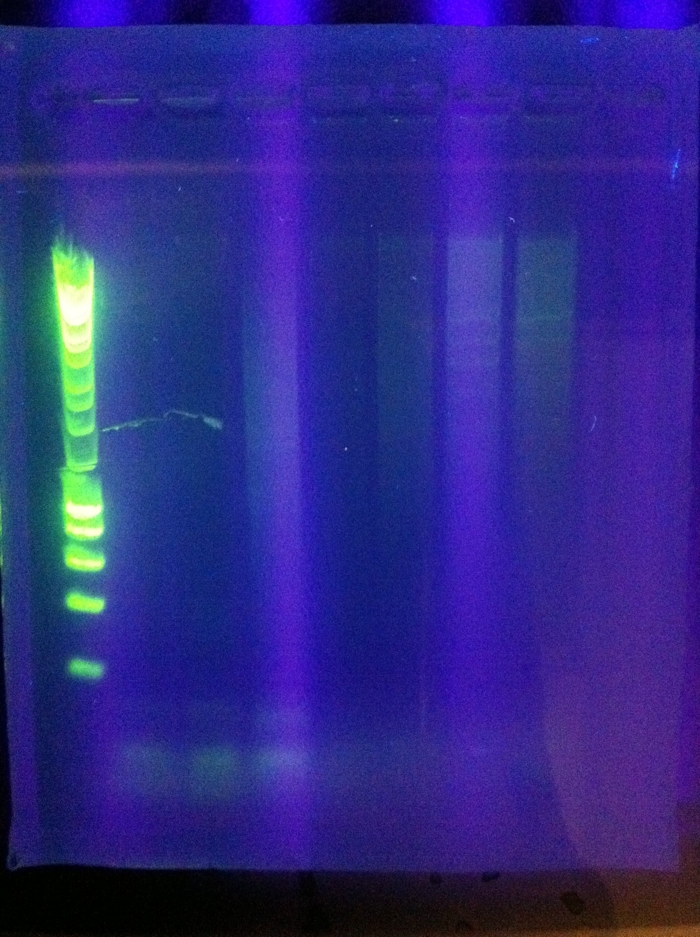
| + | <br><br>[[Image:ASU_725_gel_cas3.jpeg|300px]] | ||
| + | <br>Success for Cas3!! Extracted the band that lies around ~2500 (target is 2600). However, sequencing results were not conclusive. | ||
==Primer Round 2== | ==Primer Round 2== | ||
| - | ==Primer Round 3== | + | ===July 26, 2011=== |
| + | <b>CasABCDE</b>: 68 degree annealing temp, 2 minute elongation | ||
| + | <br><b>Cas3</b>: 62 degree annealing temp, 1:20 elongation | ||
| + | <p>Gel Results:</p> | ||
| + | CasABCDE: mostly blank | ||
| + | <br>Cas3: broad nonspecific bands around desired area | ||
| + | |||
| + | ===July 27, 2011=== | ||
| + | <b>CasABCDE (new)</b>: 64->69, three rows of 3 tubes | ||
| + | <br><b>CasABCDE (old)</b>: 63->66, three rows of 3 tubes | ||
| + | <p>Gel Results:</p> | ||
| + | [[Image:ASU_727_IMG_3952.jpg|300px]] | ||
| + | <br>new: no good | ||
| + | <br>old: mostly blank… not sure why this is. slight dimerization. | ||
| + | |||
| + | ===July 28, 2011=== | ||
| + | <b>CasABCDE (old)</b>: TDWN728B, 71 to 68, -.1/cycle, 30 cycles, 2:10 elongation | ||
| + | <br><b>CasABCDE (new)</b>: TDWN728B, 72 to 65, -.2/cycle, 35 cycles, 2:10 elongation</p> | ||
| + | <p>Gel Results:</p> | ||
| + | <br>new: no good | ||
| + | <br>old: no good | ||
| + | |||
| + | ===July 29, 2011=== | ||
| + | <b>CasABCDE</b>: 68 Touchdown from 72 to 65, -.2/cycle, 35 cycles, 2:10 elongation | ||
| + | <p>Gel Results:</p> | ||
| + | [[Image:ASU_729_PCR.jpg|300px]] | ||
| + | <br>No good | ||
| + | |||
| + | ===August 9, 2011=== | ||
| + | <b>Cas3</b>: Ran Cas3724 protocol | ||
| + | <p>Gel Results:</p> | ||
| + | [[Image: ASU_89_CasABCDE.jpg|300px]] | ||
| + | <br>new: no good | ||
| + | |||
| + | ==Primer Round 3 (CasABCDE only)== | ||
| + | ===August 4, 2011=== | ||
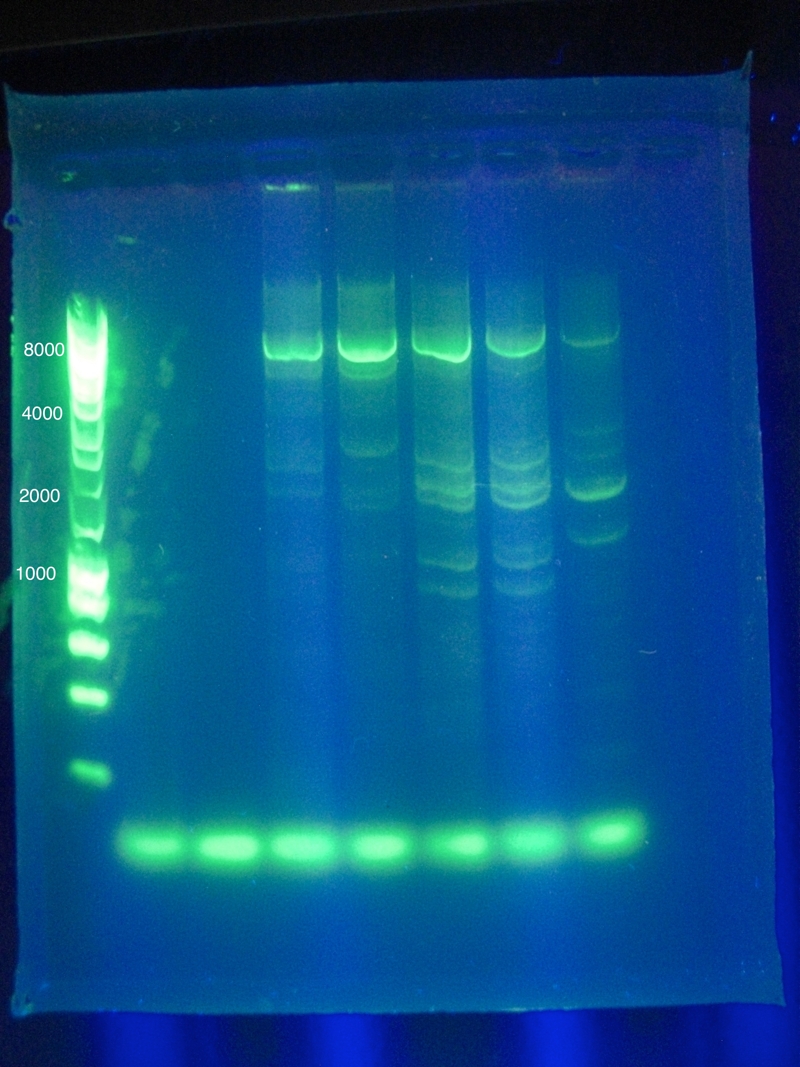
| + | <p><b>Settings</b>: 98 initial denaturation for 30 seconds, Cycle (10sec at 98deg, anneal 30 sec at 63 deg, elongate 130sec at 72deg), 35 Cycles, Extension for 450 seconds</p> | ||
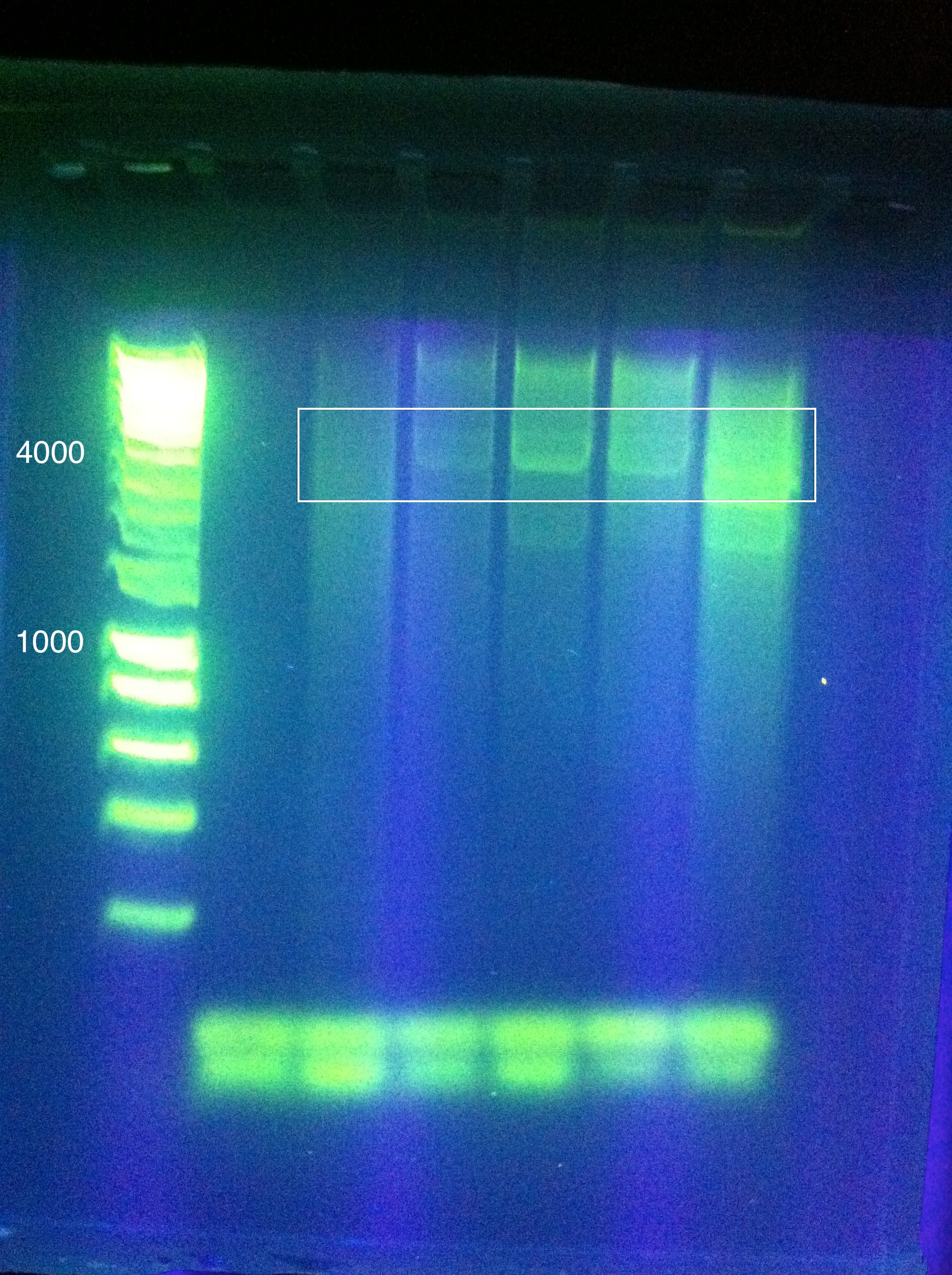
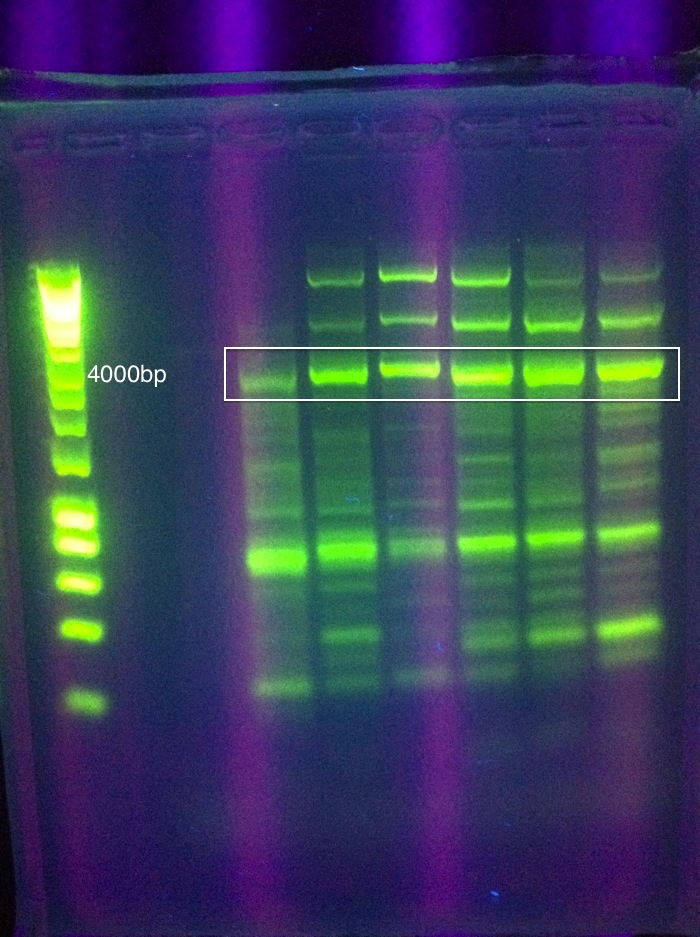
| + | [[Image:ASU_PCR_Gel_84.jpg|300px]] | ||
| + | <br>Results show a band above where we think it ought to be (6 to 8 kbp instead of 4.3) | ||
| + | However, this was extracted and an attempt at a nested PCR was made using the same settings to try and amplify this band even further. The results were blank. | ||
| + | |||
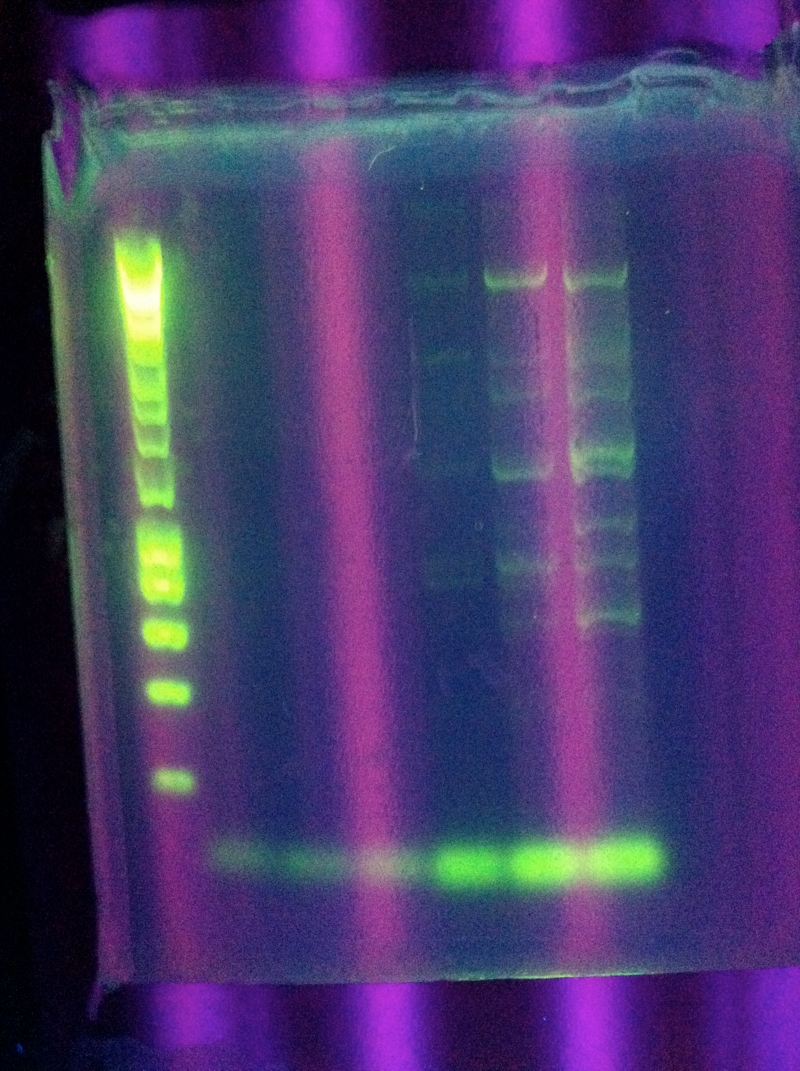
| + | ===August 8, 2011=== | ||
| + | <p><b>Settings</b>:ABCDE808, which is same as 8/4 run but only 29 cycles in the hope that the bright band from the previous try would be the only one visible.</p> | ||
| + | [[Image:ASU_88_PCR.jpg|300px]] | ||
| + | <br>Looks very similar to previous results. Again, a band above where we would like to see it. | ||
| + | |||
| + | ===August 8, 2011=== | ||
| + | <p><b>Settings</b>: Retry same protocol in thermocycler, however increase back to 35 cycles.</p> | ||
| + | [[Image: ASU_810_Cas3_1.jpg|300px]] | ||
| + | <br>Not as good as the original run, though there may be faint bands where we would like to see them. For some reason the brightest band is again somewhere between 6k and 8k (though it's a bit hard to tell because the ladder curves annoyingly at the top), instead of the desired 4300. However, we have ordered the nested PCR primers, so perhaps that will offer a better solution to getting Cas ABCDE. In the meantime, we'll focus again on Cas3 and try to extract that and get a good sequencing result so we can say definitively that we have it. | ||
| + | |||
| + | ===August 10, 2011 (Evening)=== | ||
| + | <p><b>Settings</b>: Ran another PCR for ABCDE, same settings as before but one degree higher for annealing temp. Also added DMSO.</p> | ||
| + | [[Image: ASU_810_Cas3_2.jpg|300px]] | ||
| + | <br>Similar result, less bands from DMSO but consequently much fainter bands. Dimerization still evident. | ||
| + | |||
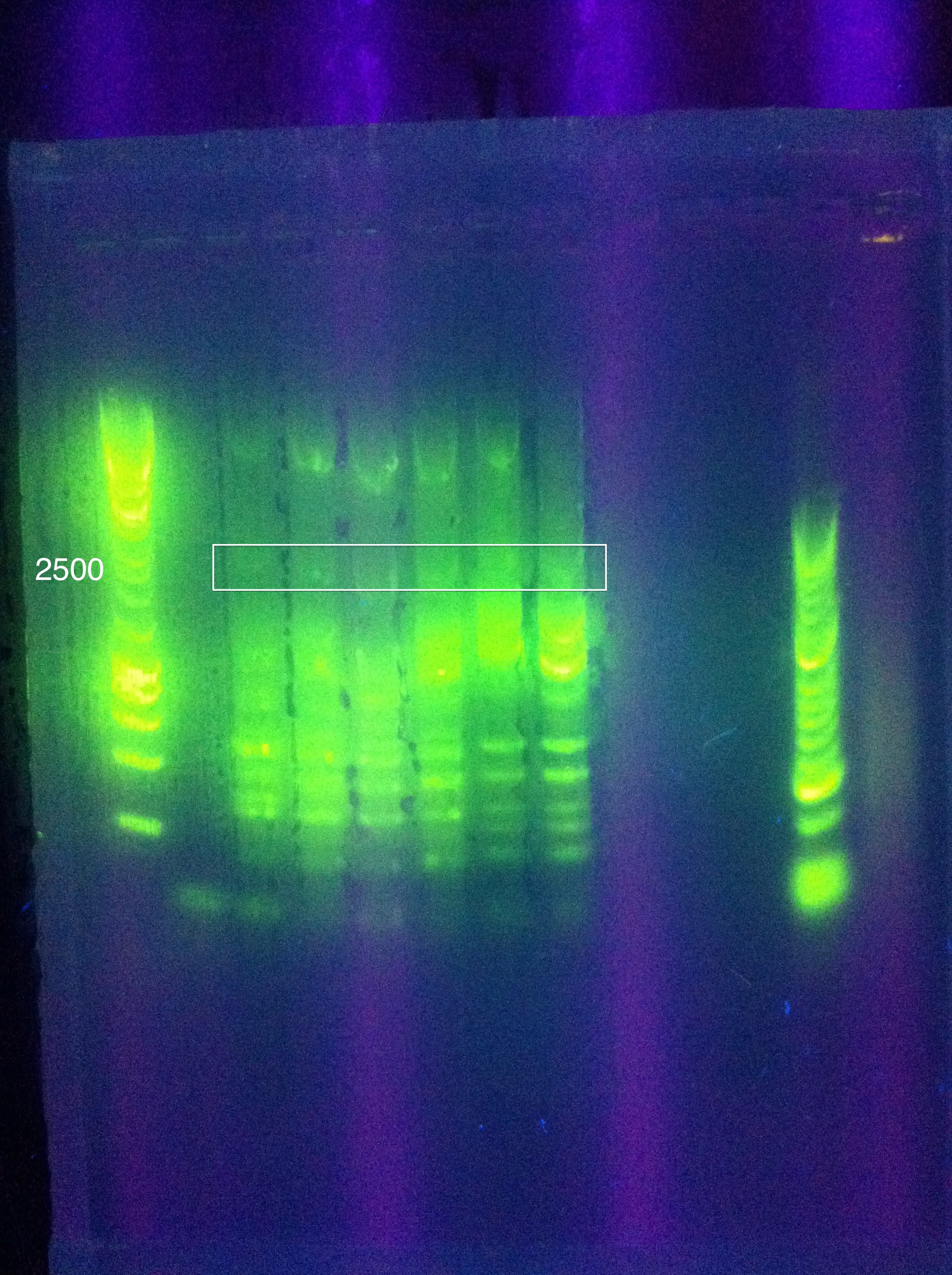
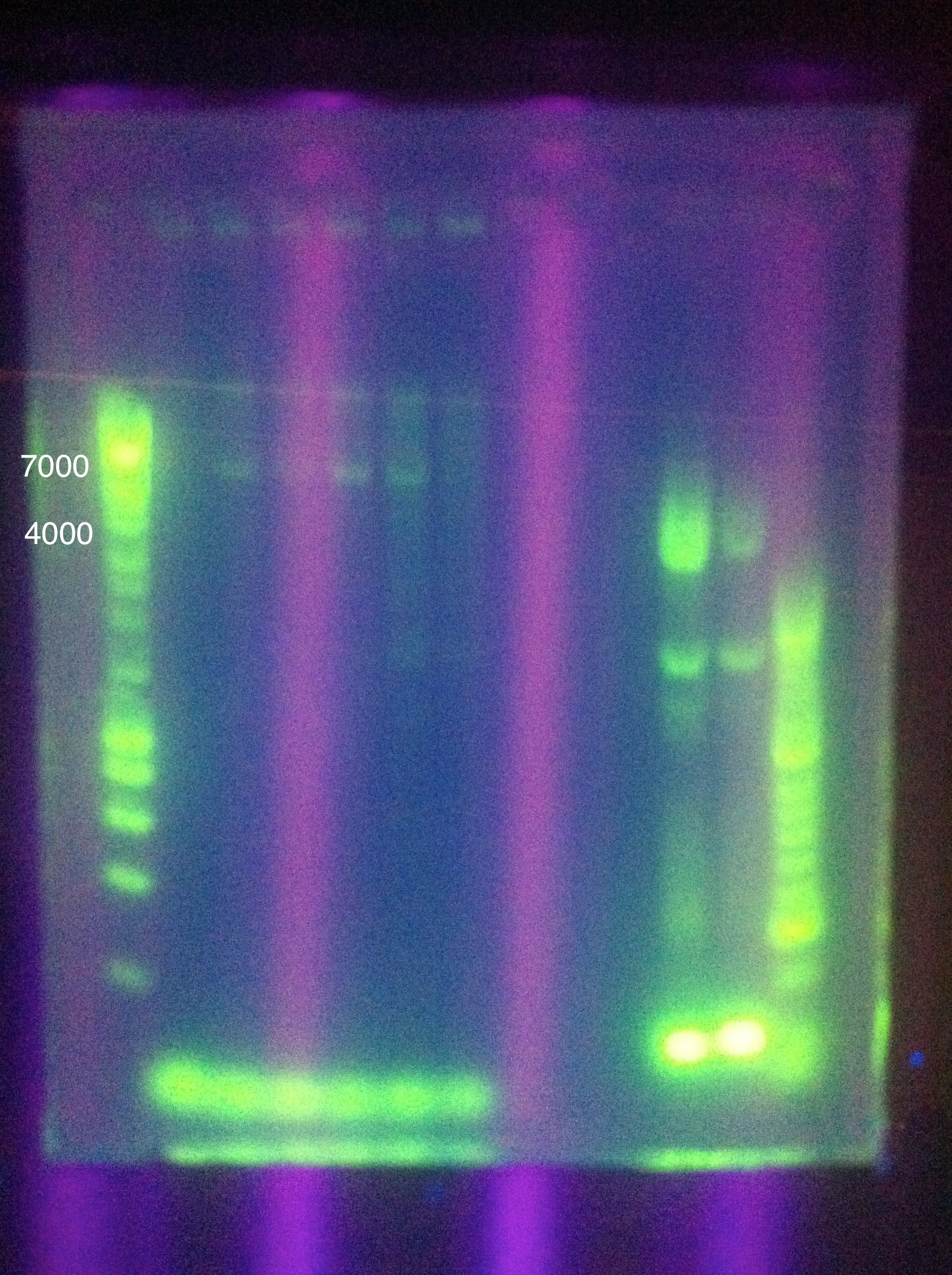
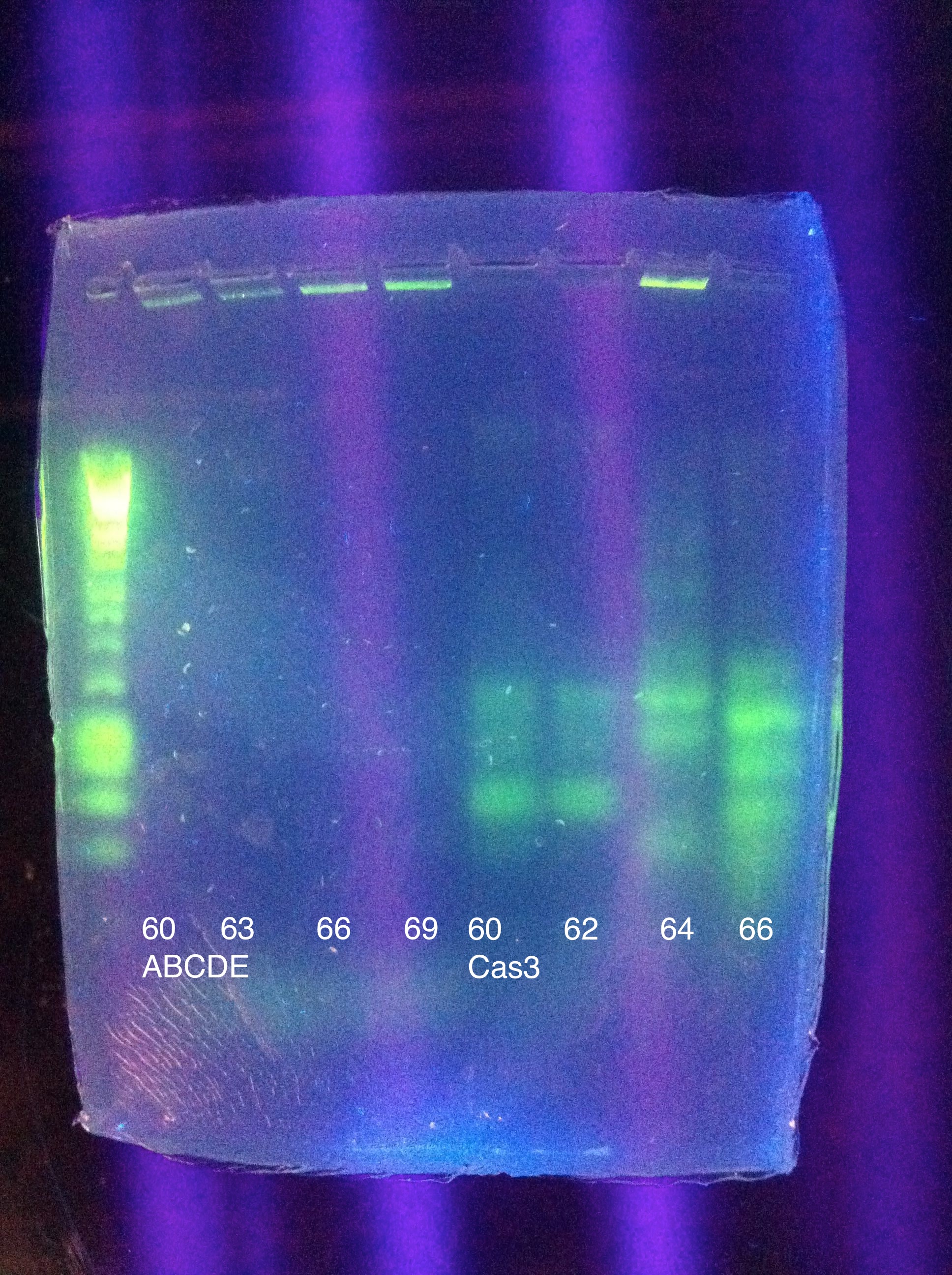
| + | ===August 11, 2011=== | ||
| + | <p>Temperature Gradient PCR</p> | ||
| + | <b>CasABCDE</b>: MgCl2 --> 2uL, 4uL, 5uL, Temp --> 60, 63, 66, 69 | ||
| + | <br><b>Cas3</b>: MgCl2 --> 2uL, 4uL, 5uL, Temp --> 60, 62, 64, 66 | ||
| + | <br>[[Image: ASU_ABCDE_Pranqster.jpeg|300px]] | ||
| + | <br>CasABCDE: No bands | ||
| + | <br>[[Image: ASU_811_Gradient_gel_result.jpeg|300px]] | ||
| + | <br>Cas3: 60 and 62 have bands less than our desired target, up to perhaps 1500; 64 had a couple of bands above this, perhaps one faint one near our 2600 target; 66 had more nonspecific amplification, and no clear bands in our target range | ||
| + | <p>Moving forward to nested PCR for ABCDE, we will see how this goes. Cas3 we'll have to keep trying…perhaps it may be worth it to try DMSO, longer elongation time, something. Maybe Taq.</p> | ||
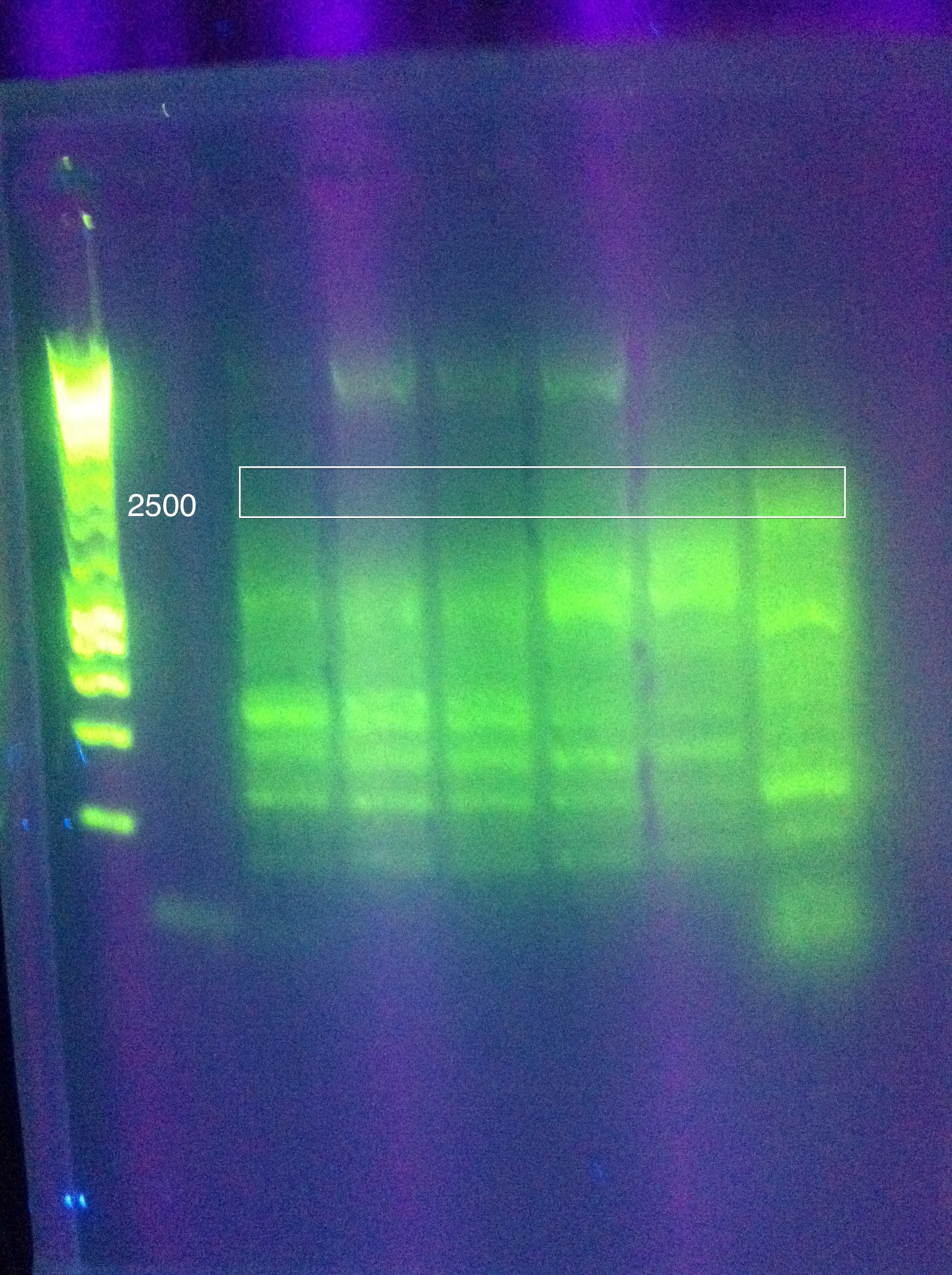
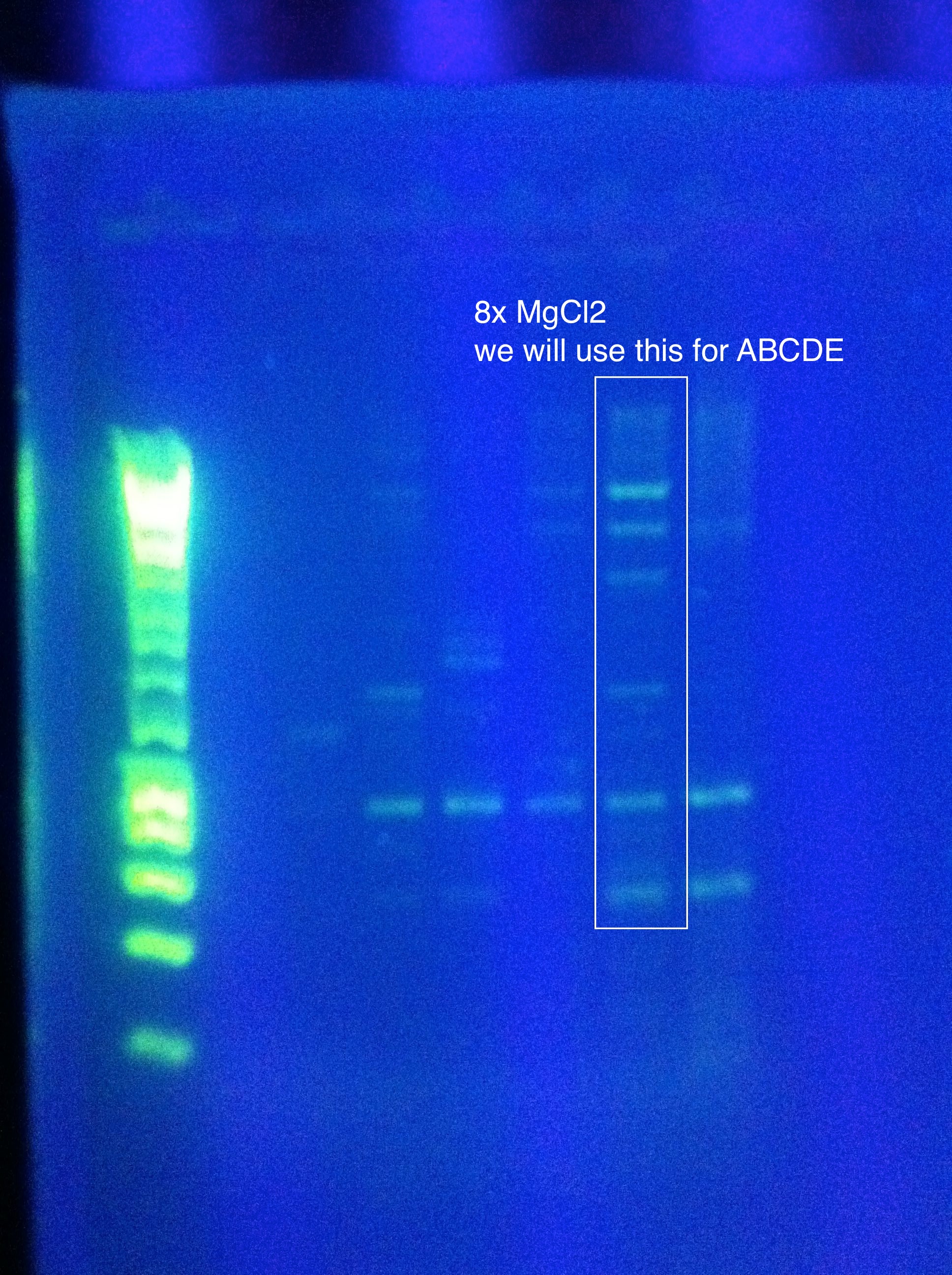
| + | ===August 12, 2011=== | ||
| + | *PCR using Nest primers for ABCDE | ||
| + | :*Tm1: 74, Tm2: 77 --> Anneal at 72 deg (higher than this is not recommended) | ||
| + | :*Used 2-step Phusion protocol | ||
| + | ::*98 deg for 30 sec | ||
| + | ::*{98deg for 10 sec, 72 deg for 2:30} X 35 cycles | ||
| + | ::*Extension for 7:30 at 72 deg | ||
| + | ::*Held at 4deg until run was cancelled | ||
| + | <br>Gel Results: | ||
| + | <br>[[Image:ASU_812_Nest.jpeg|300px]] | ||
| + | <br>The 8x MgCl2 tube has the best result | ||
| + | <br>Nice clear bands at around 600, 1k, 2k, 4k, 6k, 8k | ||
| + | <br>PLAN: use this as template for ABCDE PCR | ||
| + | <br>Use 1uL per tube (note, we don't know the concentration of the strand we want as template, so this is our best guess as to what would get us between 1pg and 10ng as recommended by the Phusion protocol for non-genomic DNA) | ||
}} <!-- close content attribute for menubar --> | }} <!-- close content attribute for menubar --> | ||
Latest revision as of 04:31, 29 September 2011
 "
"