Team:ETH Zurich/Biology/Journal
From 2011.igem.org
(Difference between revisions)
(→Week 13: 12.9.-18.9) |
(→Week 19: 24.10-30.10) |
||
| (24 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{:Team:ETH Zurich/Templates/ | + | {{:Team:ETH Zurich/Templates/HeaderNew}} |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | = Lab Journal = | |
| - | + | '''Here you can follow the progress of our project as it is coming together.''' | |
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | + | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionStart}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | { | + | |
| - | + | ||
== '''Week 1: 20.6-26.6''' == | == '''Week 1: 20.6-26.6''' == | ||
| - | |||
* First meeting | * First meeting | ||
* [[Team:ETH_Zurich/Team/Brainstorming|Brainstorming]] | * [[Team:ETH_Zurich/Team/Brainstorming|Brainstorming]] | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | |
| - | + | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
== ''' Week 2: 27.6-3.7''' == | == ''' Week 2: 27.6-3.7''' == | ||
| - | |||
* [[Team:ETH_Zurich/Team/Brainstorming|Brainstorming]] | * [[Team:ETH_Zurich/Team/Brainstorming|Brainstorming]] | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | |
| - | + | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
== ''' Week 3: 4.7-10.7''' == | == ''' Week 3: 4.7-10.7''' == | ||
| Line 51: | Line 27: | ||
* Design of several Operons for AlcR and several PCR primers | * Design of several Operons for AlcR and several PCR primers | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | |
| - | + | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
== '''Week 4: 11.7-17.7''' == | == '''Week 4: 11.7-17.7''' == | ||
| - | * Ordering of the LacI<sub>M1</sub> and | + | * Ordering of the LacI<sub>M1</sub> and codon-optimized AlcR gene |
* Making of competent DH5-α cells | * Making of competent DH5-α cells | ||
* Cloning of the AlcR-testsystem | * Cloning of the AlcR-testsystem | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | + | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionStart}} | |
== '''Week 5: 18.7-24.7''' == | == '''Week 5: 18.7-24.7''' == | ||
* First test of the AlcR-system in 96-well plates | * First test of the AlcR-system in 96-well plates | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 6: 25.7-31.7''' == | == '''Week 6: 25.7-31.7''' == | ||
* Transformation of the parts from the iGEM plates | * Transformation of the parts from the iGEM plates | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 7: 1.8-7.8''' == | == '''Week 7: 1.8-7.8''' == | ||
| Line 80: | Line 53: | ||
* Ligation and Transformation of | * Ligation and Transformation of | ||
| - | ** | + | ** P<sub>R</sub> and luxI |
| - | ** | + | ** P<sub>R</sub> and lacI |
** P<sub>lac</sub> and GFP<sub>LVA</sub> | ** P<sub>lac</sub> and GFP<sub>LVA</sub> | ||
** P<sub>lux</sub> and mCherry | ** P<sub>lux</sub> and mCherry | ||
| Line 88: | Line 61: | ||
* First meeting with Dr. Oliver Frey (Bio engineering laboratory, Prof. Andreas Hierlemann, D-BSSE ETHZ) to exchange ideas about microfluidic design | * First meeting with Dr. Oliver Frey (Bio engineering laboratory, Prof. Andreas Hierlemann, D-BSSE ETHZ) to exchange ideas about microfluidic design | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 8: 8.8-14.8''' == | == '''Week 8: 8.8-14.8''' == | ||
[[File:Lab5.jpg|right|300px]] | [[File:Lab5.jpg|right|300px]] | ||
| Line 105: | Line 77: | ||
* Improving of the testsystem | * Improving of the testsystem | ||
| - | ** Exchange of | + | ** Exchange of some rare codons in AlcR by PCR |
| - | ** His-tagging of AlcR | + | ** His-tagging of AlcR for detection in Western blot |
| - | ** | + | ** include sfGFP |
| - | * Transformation of | + | * Transformation of P<sub>R</sub> |
* Creation of competent JM101 cells | * Creation of competent JM101 cells | ||
** Check for transformation efficiency | ** Check for transformation efficiency | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
| - | + | ||
== '''Week 9: 15.8-21.8''' == | == '''Week 9: 15.8-21.8''' == | ||
[[File:IMG 6171 Kopie.JPG|right|300px]] | [[File:IMG 6171 Kopie.JPG|right|300px]] | ||
* Growth and repression test of AlcR-system with M9-medium | * Growth and repression test of AlcR-system with M9-medium | ||
* Ligation and Transformation of | * Ligation and Transformation of | ||
| - | ** | + | ** P<sub>R</sub> and lacI |
| - | ** | + | ** P<sub>R</sub> and luxI |
** P<sub>tet</sub> and CI | ** P<sub>tet</sub> and CI | ||
| - | ** | + | ** P<sub>R</sub>-lacI and terminator |
| - | ** | + | ** P<sub>R</sub>-luxI and terminator |
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 10: 22.8-28.8''' == | == '''Week 10: 22.8-28.8''' == | ||
* Transformation and Ligation | * Transformation and Ligation | ||
** P<sub>tet</sub>-CI and Terminator | ** P<sub>tet</sub>-CI and Terminator | ||
| - | *Preparation of chemically competent DH5-α and JM101 cells | + | * Preparation of chemically competent DH5-α and JM101 cells |
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 11: 29.8-4.9''' == | == '''Week 11: 29.8-4.9''' == | ||
* Transformation and Ligation | * Transformation and Ligation | ||
| Line 141: | Line 110: | ||
* Diffusion test through tube system filled with agarose and cells | * Diffusion test through tube system filled with agarose and cells | ||
* Test of the improved AlcR system | * Test of the improved AlcR system | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 12: 5.9.-11.9''' == | == '''Week 12: 5.9.-11.9''' == | ||
[[File:Lab6.JPG|right|300px]] | [[File:Lab6.JPG|right|300px]] | ||
| Line 167: | Line 135: | ||
*** P<sub>Xyl</sub> and CI | *** P<sub>Xyl</sub> and CI | ||
*** P<sub>Xyl</sub> and LacI <sub>M1</sub> | *** P<sub>Xyl</sub> and LacI <sub>M1</sub> | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 13: 12.9.-18.9''' == | == '''Week 13: 12.9.-18.9''' == | ||
[[File:IMG 6442.JPG|right|300px]] | [[File:IMG 6442.JPG|right|300px]] | ||
| - | * PCR of the | + | * PCR of the pBR322 Ori |
* PCR of the gen cluster for the upper TOL pathway | * PCR of the gen cluster for the upper TOL pathway | ||
* first test of cell printing | * first test of cell printing | ||
*Transformation and Ligation of | *Transformation and Ligation of | ||
| - | ** P<sub>const</sub>-TetR- P<sub>Tet</sub>-LacI<sub>M1</sub> and | + | ** P<sub>const</sub>-TetR- P<sub>Tet</sub>-LacI<sub>M1</sub> and P<sub>R</sub>-GFPassam |
* Western Blot of AlcR | * Western Blot of AlcR | ||
* Making of competent DH5-α cells | * Making of competent DH5-α cells | ||
* Design and construction of Psb6A5 | * Design and construction of Psb6A5 | ||
| - | + | {{:Team:ETH Zurich/Templates/SectionEnd}} | |
| - | { | + | {{:Team:ETH Zurich/Templates/SectionStart}} |
| - | + | ||
== '''Week 14: 19.9.-25.9''' == | == '''Week 14: 19.9.-25.9''' == | ||
* Characterization of LacI<sub>M1</sub> | * Characterization of LacI<sub>M1</sub> | ||
| - | |} | + | * Cloning of all biobricks into pSB1C3 |
| + | * PCR of LacI and CI | ||
| + | * wiki | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
| + | == '''Week 15: 26.9.-02.10''' == | ||
| + | [[File:IMG 6649.JPG|right|300px]] | ||
| + | * design of Pu promoter | ||
| + | * test of Pu promoter | ||
| + | * test for indol degradation | ||
| + | * Poster | ||
| + | * Preparing the presentation | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | |||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
| + | |||
| + | == '''European Jamboree 30.9-2.10''' == | ||
| + | * Sightseeing and having fun in Amsterdam | ||
| + | * Gold Medal | ||
| + | * Honorable Mention in category ''Best Model'' | ||
| + | * [https://2011.igem.org/Jamborees Jamboree] | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
| + | |||
| + | == '''Week 16: 03.10.-09.10''' == | ||
| + | * proof of concept with an arabinose inducable system | ||
| + | * cloning for the xylene system | ||
| + | * cloning of the lac-testsystem to compaire LacI<sub>M1</sub> | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
| + | == '''Week 17: 10.10.-16.10''' == | ||
| + | * cloning of the alarm-test system | ||
| + | * cloning the final system with xylene | ||
| + | * cloning the arabinose system | ||
| + | * cloning the AlcR (codon optimized) system | ||
| + | * building a channel out of different materials | ||
| + | * Design a assay for testing the alarm system | ||
| + | * Sender receiver test on plates | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
| + | |||
| + | == '''Week 18: 17.10.-23.10''' == | ||
| + | * Cloning | ||
| + | * inactivating of the kanamycin casette of BW27749 to get BW27783 | ||
| + | * Make competent BW27783 and DH5-α cells | ||
| + | * Sender receiver test on plates with sterile filtrated supernatant | ||
| + | * Sender an receiver test in tube | ||
| + | * Dose response experiment for xylene | ||
| + | * Dose response experiment for acetaldehyde | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
| + | |||
| + | == '''Week 19: 24.10-30.10''' == | ||
| + | * Cloning | ||
| + | * Microscope experiments of the channel with and without degradation | ||
| + | * Alarm test with AHL | ||
| + | * Dose response experiment for acetaldehyde | ||
| + | * Dose response experiment for arabinose | ||
| + | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
| + | {{:Team:ETH Zurich/Templates/HeaderNewEnd}} | ||
Latest revision as of 19:50, 27 October 2011

Lab Journal
Here you can follow the progress of our project as it is coming together.
Week 3: 4.7-10.7
- Working on the design of the system
- Design of several Operons for AlcR and several PCR primers
Week 4: 11.7-17.7
- Ordering of the LacIM1 and codon-optimized AlcR gene
- Making of competent DH5-α cells
- Cloning of the AlcR-testsystem
Week 5: 18.7-24.7
- First test of the AlcR-system in 96-well plates
Week 6: 25.7-31.7
- Transformation of the parts from the iGEM plates
Week 7: 1.8-7.8
- Growth test of DH5-α with AlcR-system in flasks
- Ligation and Transformation of
- PR and luxI
- PR and lacI
- Plac and GFPLVA
- Plux and mCherry
- Ptet and CI -had to be redone because of point mutation in the primer (week 9)
- Pconst and luxR
- First meeting with Dr. Oliver Frey (Bio engineering laboratory, Prof. Andreas Hierlemann, D-BSSE ETHZ) to exchange ideas about microfluidic design
Week 8: 8.8-14.8
- Synthesized LacIM1 Gen arrived
- Ligation and Transformation of
- Plac-GFPLVA and Terminator
- Plux-mCherry and Terminator
- Pconst-luxR and Terminator
- Plac-GFPLVA-Terminator and Plux-mCherry-Terminator on one plasmid
- Ptet and LacIM1
- Ptet-LacIM1 and Terminator
- Improving of the testsystem
- Exchange of some rare codons in AlcR by PCR
- His-tagging of AlcR for detection in Western blot
- include sfGFP
- Transformation of PR
- Creation of competent JM101 cells
- Check for transformation efficiency
Week 9: 15.8-21.8
- Growth and repression test of AlcR-system with M9-medium
- Ligation and Transformation of
- PR and lacI
- PR and luxI
- Ptet and CI
- PR-lacI and terminator
- PR-luxI and terminator
Week 10: 22.8-28.8
- Transformation and Ligation
- Ptet-CI and Terminator
- Preparation of chemically competent DH5-α and JM101 cells
Week 11: 29.8-4.9
- Transformation and Ligation
- Ptet-CI and Terminator
- Diffusion test through tube system filled with agarose and cells
- Test of the improved AlcR system
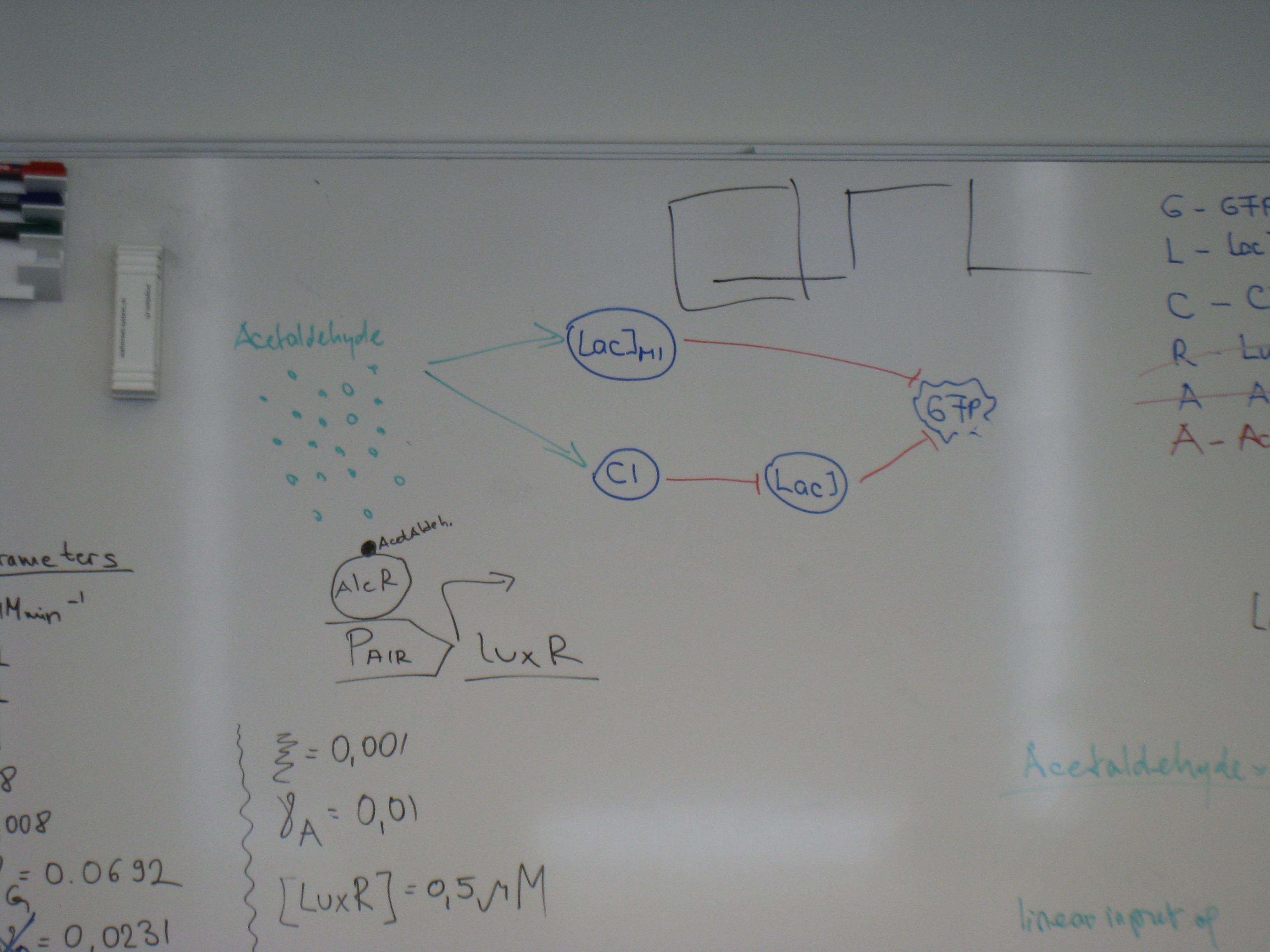
Week 12: 5.9.-11.9
- Design of a system to characterize our new biobrick LacIM1
- Transformation and Ligation
- PTet and LacI
- PLac and GFP assam
- Transformation and Ligation
- Design of an additional construct to test the band pass filter
- Transformation and Ligation
- Pconst and TetRLVA
- Transformation and Ligation
- Growth-test of E. coli in M9 with reduced Ampicillin concentration
- AlcR test with reduced Ampicillin concentration
- Testdigest of λP-luxI-Terminator
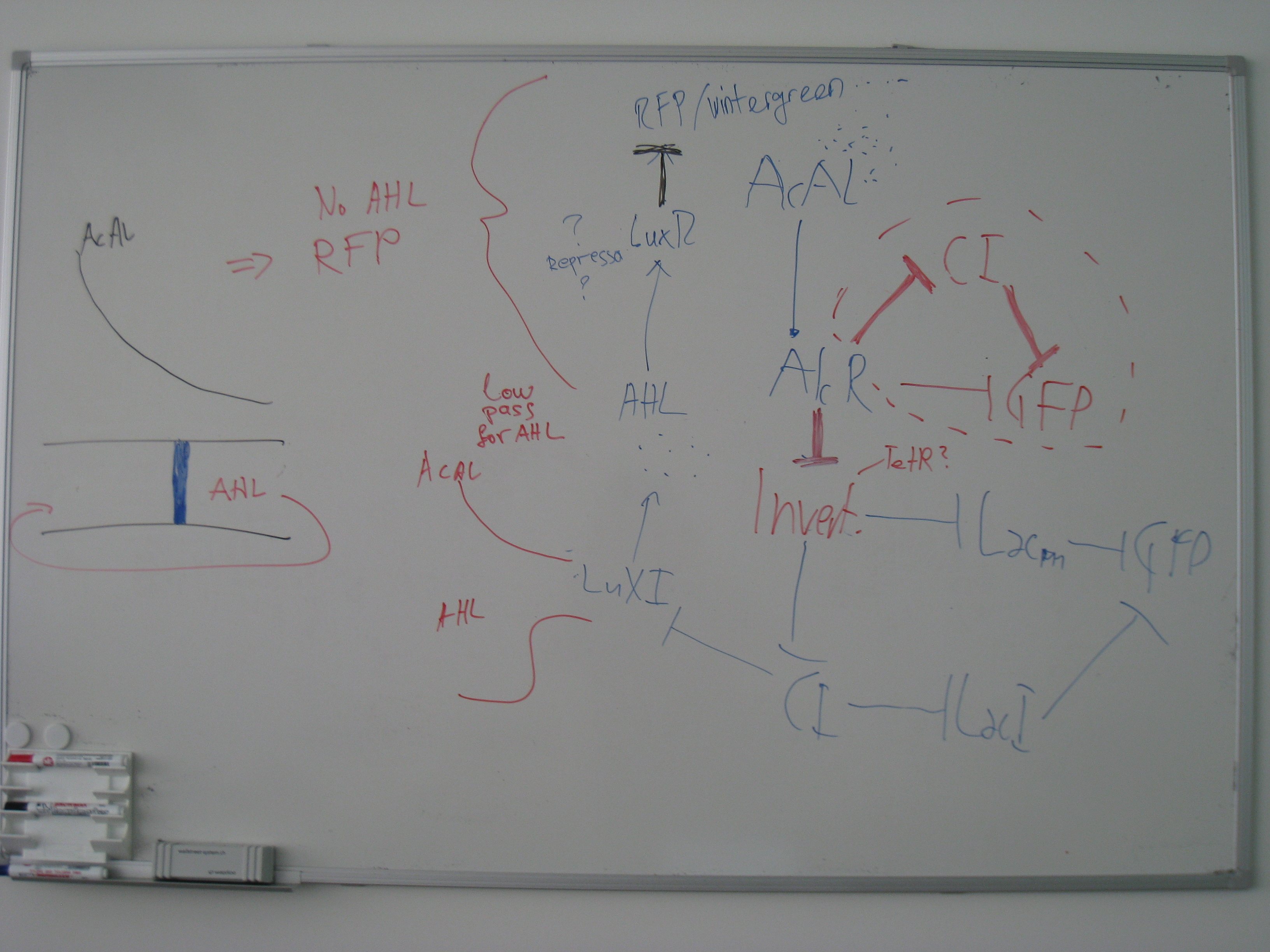
- Alternative Sensor system: Xylene system
- Transformation and Ligation
- Pconst and XylR
- PXyl and CI
- PXyl and LacI M1
- Transformation and Ligation
Week 13: 12.9.-18.9
- PCR of the pBR322 Ori
- PCR of the gen cluster for the upper TOL pathway
- first test of cell printing
- Transformation and Ligation of
- Pconst-TetR- PTet-LacIM1 and PR-GFPassam
- Western Blot of AlcR
- Making of competent DH5-α cells
- Design and construction of Psb6A5
Week 14: 19.9.-25.9
- Characterization of LacIM1
- Cloning of all biobricks into pSB1C3
- PCR of LacI and CI
- wiki
Week 15: 26.9.-02.10
- design of Pu promoter
- test of Pu promoter
- test for indol degradation
- Poster
- Preparing the presentation
European Jamboree 30.9-2.10
- Sightseeing and having fun in Amsterdam
- Gold Medal
- Honorable Mention in category Best Model
- Jamboree
Week 16: 03.10.-09.10
- proof of concept with an arabinose inducable system
- cloning for the xylene system
- cloning of the lac-testsystem to compaire LacIM1
Week 17: 10.10.-16.10
- cloning of the alarm-test system
- cloning the final system with xylene
- cloning the arabinose system
- cloning the AlcR (codon optimized) system
- building a channel out of different materials
- Design a assay for testing the alarm system
- Sender receiver test on plates
Week 18: 17.10.-23.10
- Cloning
- inactivating of the kanamycin casette of BW27749 to get BW27783
- Make competent BW27783 and DH5-α cells
- Sender receiver test on plates with sterile filtrated supernatant
- Sender an receiver test in tube
- Dose response experiment for xylene
- Dose response experiment for acetaldehyde
Week 19: 24.10-30.10
- Cloning
- Microscope experiments of the channel with and without degradation
- Alarm test with AHL
- Dose response experiment for acetaldehyde
- Dose response experiment for arabinose
 "
"