Team:Bielefeld-Germany/Results/S-Layer/Guide/6
From 2011.igem.org
| (17 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Bielefeld_2011_Header}} | {{Bielefeld_2011_Header}} | ||
| - | + | <html><img src="https://static.igem.org/mediawiki/2011/2/28/Bielefeld-header-guide.png"/><p></p></html> | |
=His-tag affinity chromatography= | =His-tag affinity chromatography= | ||
| - | + | [[Image:IGEM-Bielefeld2011-Histraps.JPG|280px|thumb|left|We used HisTrap FF crude columns by GE Healthcare.]] | |
| + | [[Image:IGEM-Bielefeld2011-AEKTA.JPG|280px|thumb|left|GE Healthcare ÄKTAprime™ plus chromatography.]] | ||
| + | [[Image:IGEM-Bielefeld2011-Saeulen.JPG|280px|thumb|left|Some chromatography columns.]] | ||
| + | |||
| + | <br style="clear: both" /> | ||
| + | |||
| + | [http://lmgtfy.com/?q=his-tag Polyhistidine tags] are used for easy purification. They bind to cobalt or nickel ions which are normally immobilized on chromatography beads or in spin columns. We used a 1 mL ''HisTrap FF crude'' by [http://www.gehealthcare.com/ GE Healthcare] in combination with a [http://www.gelifesciences.com/aptrix/upp01077.nsf/Content/aktadesign_platform~akta_primeplus GE Healthcare ÄKTAprime™ plus] FPLC device. Alternatively, you can just use a syringe which fits to [http://www.gelifesciences.com/aptrix/upp01077.nsf/Content/Products?OpenDocument&moduleid=165904 these columns]. We did not test commercial spin column kits for his-tag purification but perhaps it even works with these (offered e.g. by Qiagen). After binding of the proteins to the column and removal from the filtered cell lysate, they were eluted with 50 mM imidazol. This fraction was collected and dialysed against ddH<sub>2</sub>O at 4 °C for 18 h using dialysis tubes obtained from [http://www.carlroth.com/catalogue/catalogue.do;jsessionid=36FBA09A4B8B2D86BA4321073514B5FA?favOid=00000009000256d300020023&act=showBookmark&lang=de-de&market=DE Roth] with a 6 kDa cut-off. | ||
| + | |||
| + | |||
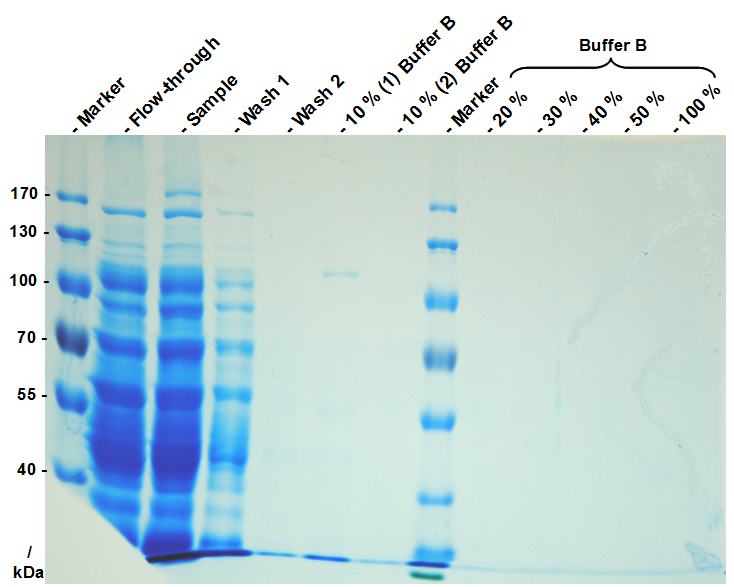
| + | [[Image:Bielefeld Germany_322_Histrap.png|280px|thumb|left|SDS-PAGE from purification.]] | ||
| + | [[Image:IGEM-Bielefeld2011-Nanodropitlikeithot.JPG|280px|thumb|left|A NanoDrop spectrophotometer.]] | ||
| + | [[Image:IGEM-Bielefeld2011-BCA.JPG|280px|thumb|left|A BCA assay.]] | ||
| + | |||
| + | <br style="clear: both" /> | ||
| + | |||
| + | After dialysis, you have purified S-layer fusion protein monomers which are ready to assemble (the so-called monomer solution). Check the protein concentration for example via BCA assay or NanoDrop and dilute to 1 mg mL<sup>-1</sup>. Check the purity of your monomer solution by SDS-PAGE as well. | ||
| + | |||
| + | Now you have your S-layer monomer solution and can start [[Team:Bielefeld-Germany/Results/S-Layer/Guide/7 | immobilization and recrystallization to build functionalized nanobiotechnological surfaces]]. | ||
| + | |||
| + | We have tested a lot of purification strategies - look them up in our [[Team:Bielefeld-Germany/Protocols/Downstream-processing | upstream and downstream protocols]]. | ||
| - | + | <html> | |
| + | <div style="text-align:center; width:535px; margin-left:auto; margin-right:auto;"> | ||
| + | <img id="Image-Maps_8201110281849269" src="https://static.igem.org/mediawiki/2011/0/0b/Bielefeld-Germany-2011-Navigationbar.png" usemap="#Image-Maps_8201110281849269" border="0" width="535" height="82" alt="" /> | ||
| + | <map id="_Image-Maps_8201110281849269" name="Image-Maps_8201110281849269"> | ||
| + | <area shape="rect" coords="22,12,170,62" href="https://2011.igem.org/Team:Bielefeld-Germany/Results/S-Layer/Guide/5" alt="Previous page" title="Previous page" /> | ||
| + | <area shape="rect" coords="182,7,330,57" href="https://2011.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Results/S-Layer/Guide" alt="Return to beginning" title="Return to beginning" /> | ||
| + | <area shape="rect" coords="353,10,501,60" href="https://2011.igem.org/Team:Bielefeld-Germany/Results/S-Layer/Guide/7" alt="Next page" title="Next page" /> | ||
| + | </map> | ||
| + | </div> | ||
| + | </html> | ||
Latest revision as of 02:47, 29 October 2011


His-tag affinity chromatography
[http://lmgtfy.com/?q=his-tag Polyhistidine tags] are used for easy purification. They bind to cobalt or nickel ions which are normally immobilized on chromatography beads or in spin columns. We used a 1 mL HisTrap FF crude by [http://www.gehealthcare.com/ GE Healthcare] in combination with a [http://www.gelifesciences.com/aptrix/upp01077.nsf/Content/aktadesign_platform~akta_primeplus GE Healthcare ÄKTAprime™ plus] FPLC device. Alternatively, you can just use a syringe which fits to [http://www.gelifesciences.com/aptrix/upp01077.nsf/Content/Products?OpenDocument&moduleid=165904 these columns]. We did not test commercial spin column kits for his-tag purification but perhaps it even works with these (offered e.g. by Qiagen). After binding of the proteins to the column and removal from the filtered cell lysate, they were eluted with 50 mM imidazol. This fraction was collected and dialysed against ddH2O at 4 °C for 18 h using dialysis tubes obtained from [http://www.carlroth.com/catalogue/catalogue.do;jsessionid=36FBA09A4B8B2D86BA4321073514B5FA?favOid=00000009000256d300020023&act=showBookmark&lang=de-de&market=DE Roth] with a 6 kDa cut-off.
After dialysis, you have purified S-layer fusion protein monomers which are ready to assemble (the so-called monomer solution). Check the protein concentration for example via BCA assay or NanoDrop and dilute to 1 mg mL-1. Check the purity of your monomer solution by SDS-PAGE as well.
Now you have your S-layer monomer solution and can start immobilization and recrystallization to build functionalized nanobiotechnological surfaces.
We have tested a lot of purification strategies - look them up in our upstream and downstream protocols.

 "
"