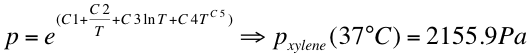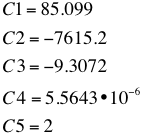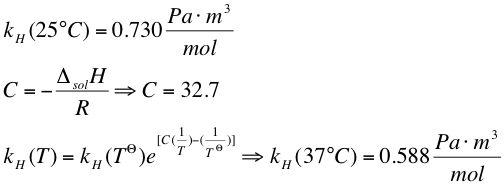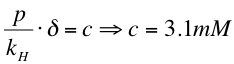Team:ETH Zurich/xylene
From 2011.igem.org
(Difference between revisions)
(→References) |
(→How we calculated the concentration of m-xylene in the medium...) |
||
| (One intermediate revision not shown) | |||
| Line 62: | Line 62: | ||
|} | |} | ||
<br clear="all" /> | <br clear="all" /> | ||
| - | [[File:ETH Back.png|200px|right|link=Team:ETH_Zurich/Biology/Validation# | + | [[File:ETH Back.png|200px|right|link=Team:ETH_Zurich/Biology/Validation#Results_of_BBa_K625003_characterization]] |
{{:Team:ETH Zurich/Templates/SectionEnd}} | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
Latest revision as of 21:54, 28 October 2011
How we calculated the concentration of m-xylene in the medium...
1. We assumed that 5 ml xylene is enough to saturate the gas phase in the Erlenmeyer flask.
- The vapor pressure was calculated at 37 °C:
- with the following C1-C5 values:
2. The Henry´s constant at 37 °C can be calculated by the Van´t Hoff equation from the literature value at 25°C
3. By Henry´s law we calculated the concentration of xylene in the medium
4. For the oil/xylene mixtures we assumed that paraffin oil has a vapor pressure of 0 and used Raoult's law
| xylene in oil in % | concentration of xylene in LB [mM] |
| 100 | 3.1 |
| 50 | 1.55 |
| 25 | 0.775 |
| 15 | 0.465 |
| 10 | 0.31 |
| 7.5 | 0.233 |
| 5 | 0.155 |
| 2.5 | 0.078 |
| 1 | 0.031 |
| 0.75 | 0.023 |
| 0.5 | 0.016 |
| 0 | 0 |
References
[1] Perry’s Chemical Engineers Handbook 8th Edition
[2] CRC Handbook of Chemistry and Physics, 92nd Edition, p.5-189
 "
"