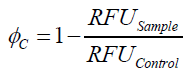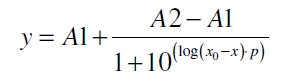Team:Bielefeld-Germany/Results/S-Layer/SgsE
From 2011.igem.org
(→Summary of advances) |
(→Summary of advances) |
||
| Line 7: | Line 7: | ||
- Isolation and purification of the inclusion bodies using detergents, ultra- and diafiltration. Providing of water-soluble fusion protein monomers for recrsytallisation and coating through dialyzation. (See: [[Team:Bielefeld-Germany/Results/S-Layer/SgsE#Purification_of_SgsE_fusion_protein|Purification of SgsE fusion protein inclusion bodies]]). | - Isolation and purification of the inclusion bodies using detergents, ultra- and diafiltration. Providing of water-soluble fusion protein monomers for recrsytallisation and coating through dialyzation. (See: [[Team:Bielefeld-Germany/Results/S-Layer/SgsE#Purification_of_SgsE_fusion_protein|Purification of SgsE fusion protein inclusion bodies]]). | ||
| - | - Characterization of immobilization behaviour of proteins coated to silica beads through measurement of fluorescence of supernatant, the wash fraction and the beads. (See: [[Team:Bielefeld-Germany/Results/S-Layer/SgsE#Immobilization_behaviour|Immobilization behaviour]]). Immobilization kinetics to determine the optimal bead to protein ratio (See: [[Team:Bielefeld-Germany/Results/S-Layer/SgsE#Optimal_bead_to_protein_ratio_for_immobilization|bead to protein ratio]]). A good silica bead conentration for immobilization of 100 µg protein could be calculated to be around 150 - 200 mg mL<sup>-1</sup>. | + | - Characterization of immobilization behaviour of proteins coated to silica beads through measurement of fluorescence of supernatant, the wash fraction and the beads. (See: [[Team:Bielefeld-Germany/Results/S-Layer/SgsE#Immobilization_behaviour|Immobilization behaviour]]). |
| + | |||
| + | - Immobilization kinetics to determine the optimal bead to protein ratio (See: [[Team:Bielefeld-Germany/Results/S-Layer/SgsE#Optimal_bead_to_protein_ratio_for_immobilization|bead to protein ratio]]). Data could be fitted to a dose-response function. A good silica bead conentration for immobilization of 100 µg protein could be calculated to be around 150 - 200 mg mL<sup>-1</sup>. | ||
=SgsE from ''Geobacillus stearothermophilus'' NRS 2004/3a= | =SgsE from ''Geobacillus stearothermophilus'' NRS 2004/3a= | ||
Revision as of 16:36, 27 October 2011


Contents |
Summary of advances
- Characterization of the expression of [http://partsregistry.org/wiki/index.php/Part:BBa_K525305 K525305], a fusion of the sgsE gene ([http://partsregistry.org/wiki/index.php/Part:BBa_K525303 K525303]) with mCitrine ([http://partsregistry.org/Part:BBa_J18931 J18931]). Expression was observed through measurement of fluorescence intensity and optical density (See: Expression in E. coli).
- Isolation and purification of the inclusion bodies using detergents, ultra- and diafiltration. Providing of water-soluble fusion protein monomers for recrsytallisation and coating through dialyzation. (See: Purification of SgsE fusion protein inclusion bodies).
- Characterization of immobilization behaviour of proteins coated to silica beads through measurement of fluorescence of supernatant, the wash fraction and the beads. (See: Immobilization behaviour).
- Immobilization kinetics to determine the optimal bead to protein ratio (See: bead to protein ratio). Data could be fitted to a dose-response function. A good silica bead conentration for immobilization of 100 µg protein could be calculated to be around 150 - 200 mg mL-1.
SgsE from Geobacillus stearothermophilus NRS 2004/3a
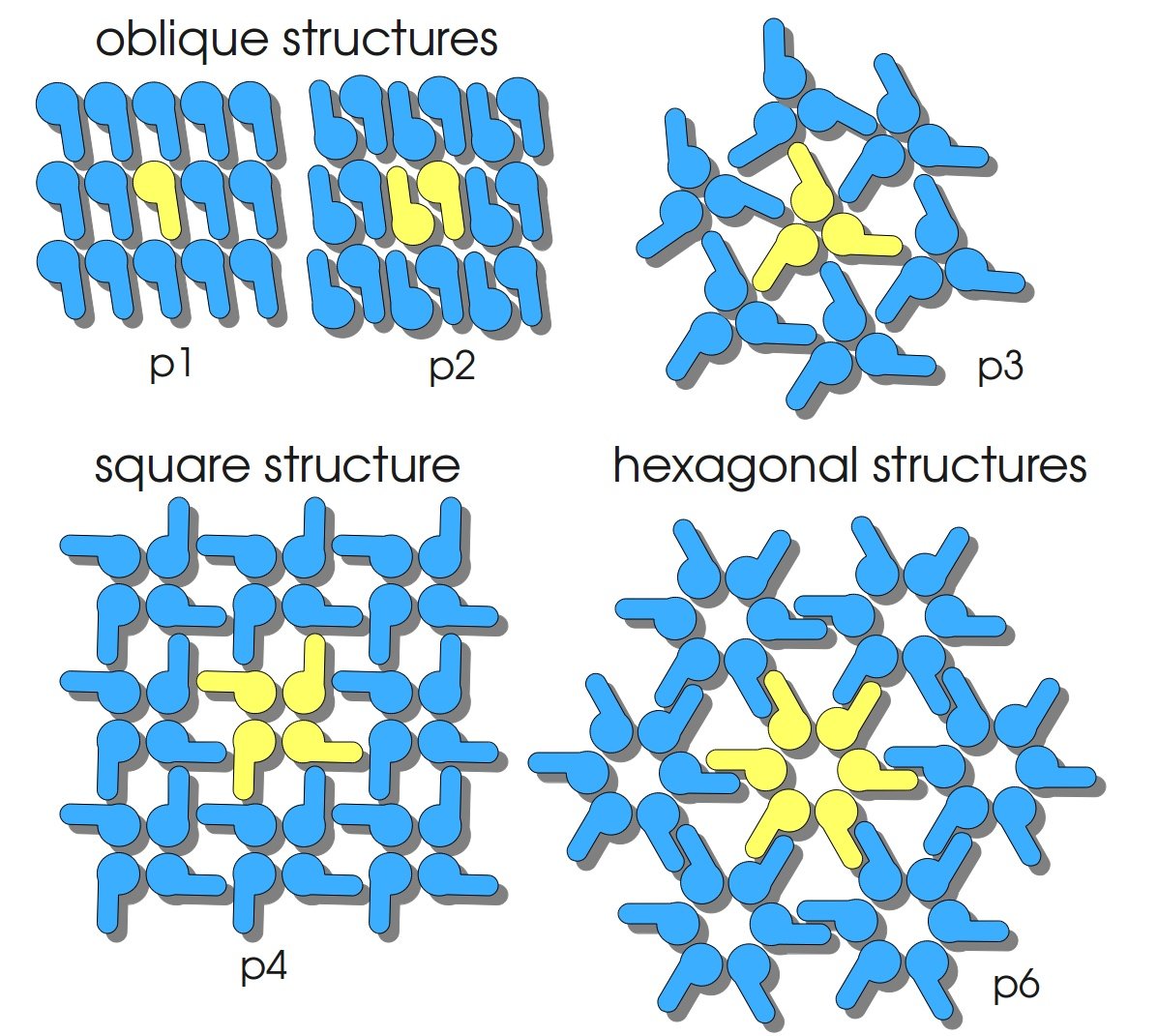
SgsE monomers are naturally assembled in a lattice with oblique symmetry (p2) (see fig. 2) exhibiting a well-defined periodicity and distances of 9.4 – 11.6 nm between the proteinaceous subunits. The S-layer protein SgsE of Geobacillus stearothermophilus NRS 2004/3a consists of 903 amino acids, including a 30 amino acid signal peptide (SLH-domain) at the amino-terminus. The carboxy-terminal part of SgsE is the larger part of the protein, encoding the self-assembly information. The protein is formed by the sgsE gene, has a calculated mass of 93.7 kDa and an isoelectric point of 6.1. When isolated SgsE maintains its ability to self-assemble, and depending on salt concentration, duration of dialysis to remove the detergent and its amino acid sequence it builds up five types of self-assembly products. These products are formed like flat sheets and cylinders ([http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer et al., 2007]).
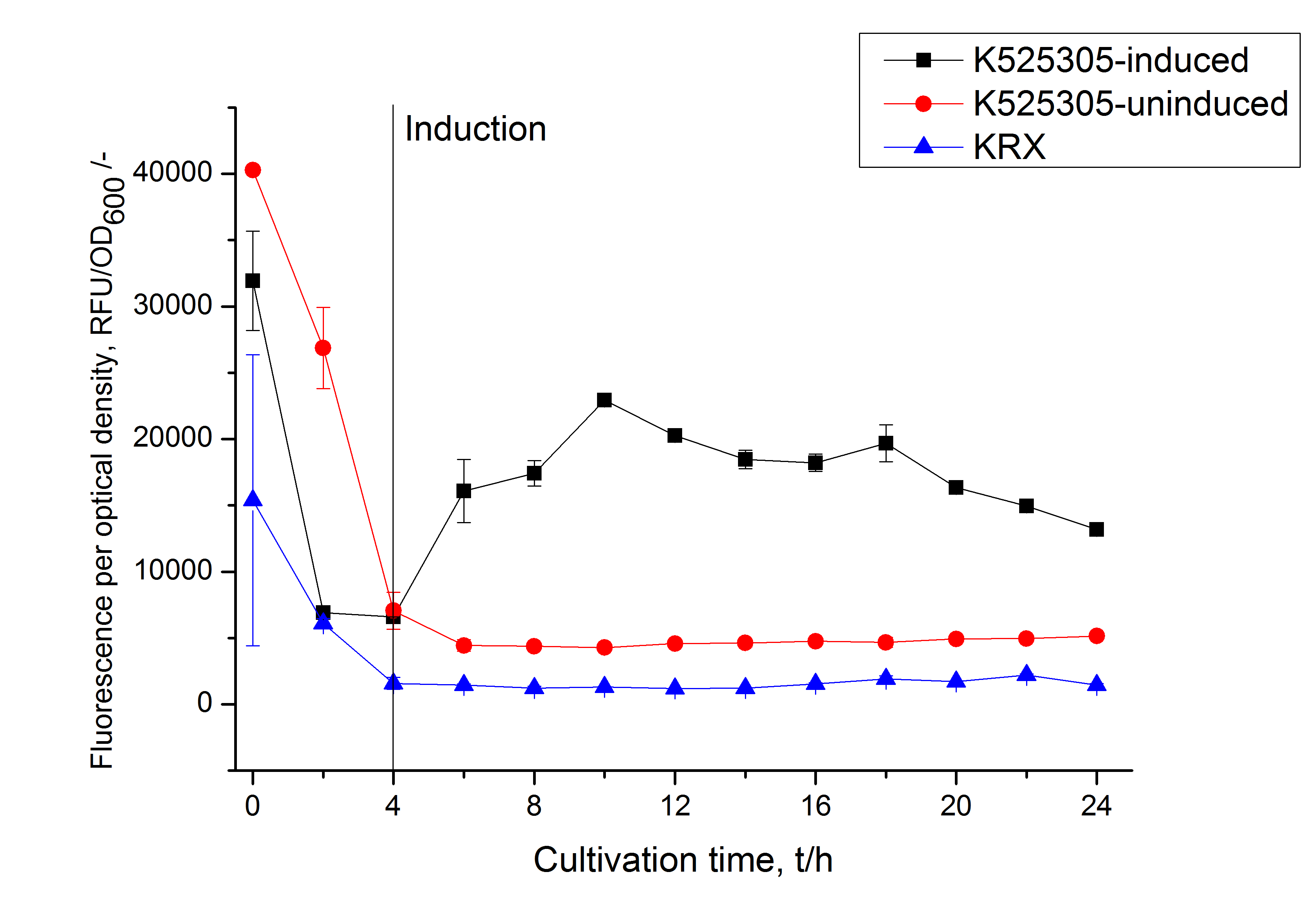
Expression in E. coli
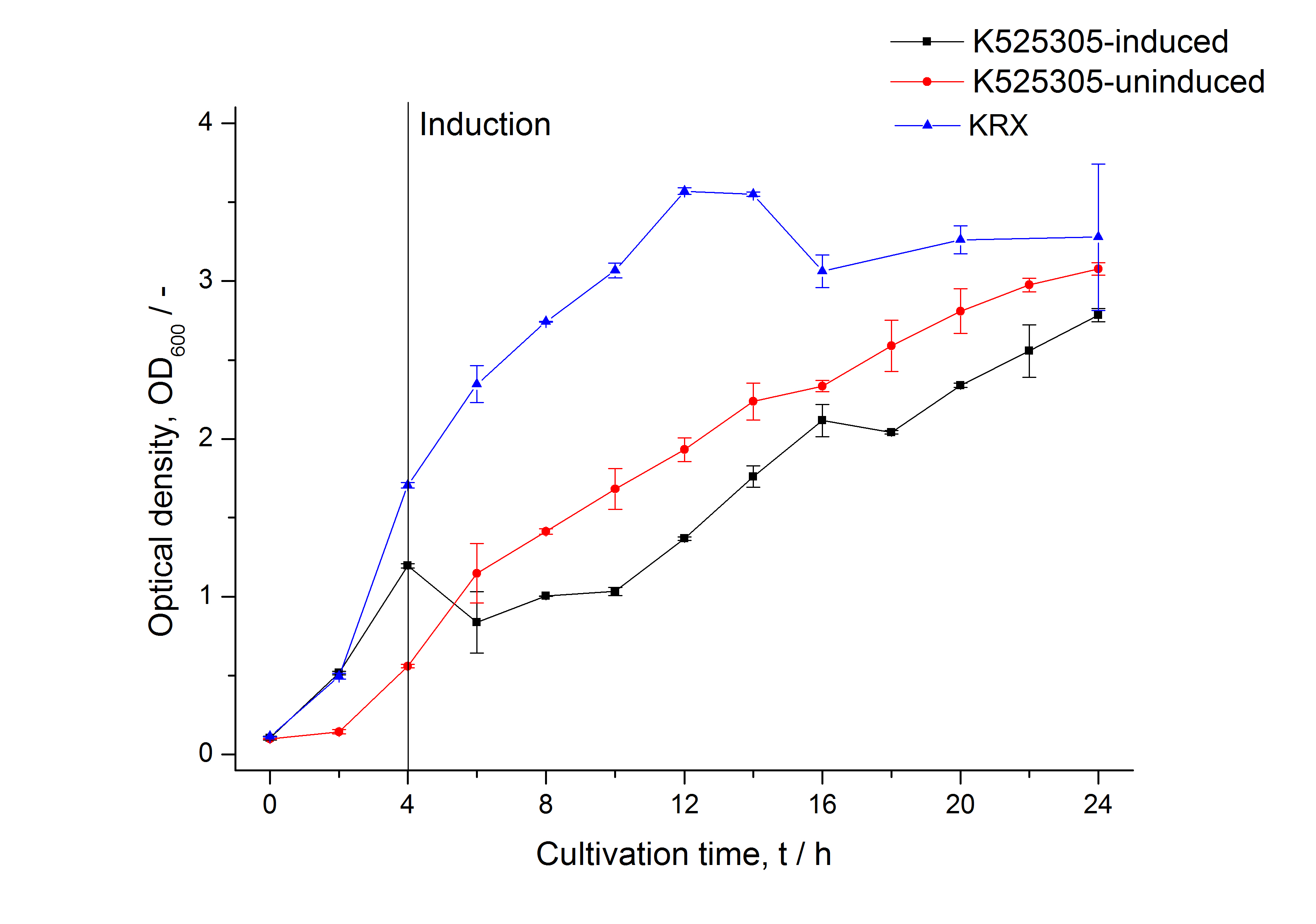
The SgsE gene under the control of a T7 / lac promoter (<partinfo>K525303</partinfo>) was fused to mCitrine ([http://partsregistry.org/Part:BBa_J18931 BBa_J18931]) using Freiburg BioBrick assembly for characterization experiments.
The SgsE|mCitrine fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0.1 % L-rhamnose and 1 mM IPTG using the autinduction protocol by Promega.
Purification of SgsE fusion protein
As observed in the analysis of the cultivations with expression of SgsE | mCitrine fusion proteins, these proteins form inclusion bodies in E. coli. Inclusion bodies have the advantage that they are relatively easy to clean-up and are resistant to proteases. So the first purification step is to isolate and solubilize the inclusion bodies. This step is followed by two filtrations (300 kDa UF and 100 kDa DF/UF) to further concentrate and purify the S-layer proteins. After the filtrations, the remaining protein solution is dialyzed against ddH2O for 18 h at 4 °C in the dark. The dialysis leads to a precipitation of the water-insoluble proteins. After centrifugation of the dialysate the water-soluble S-layer monomers remain in the supernatant and can be used for recrystallization experiments.
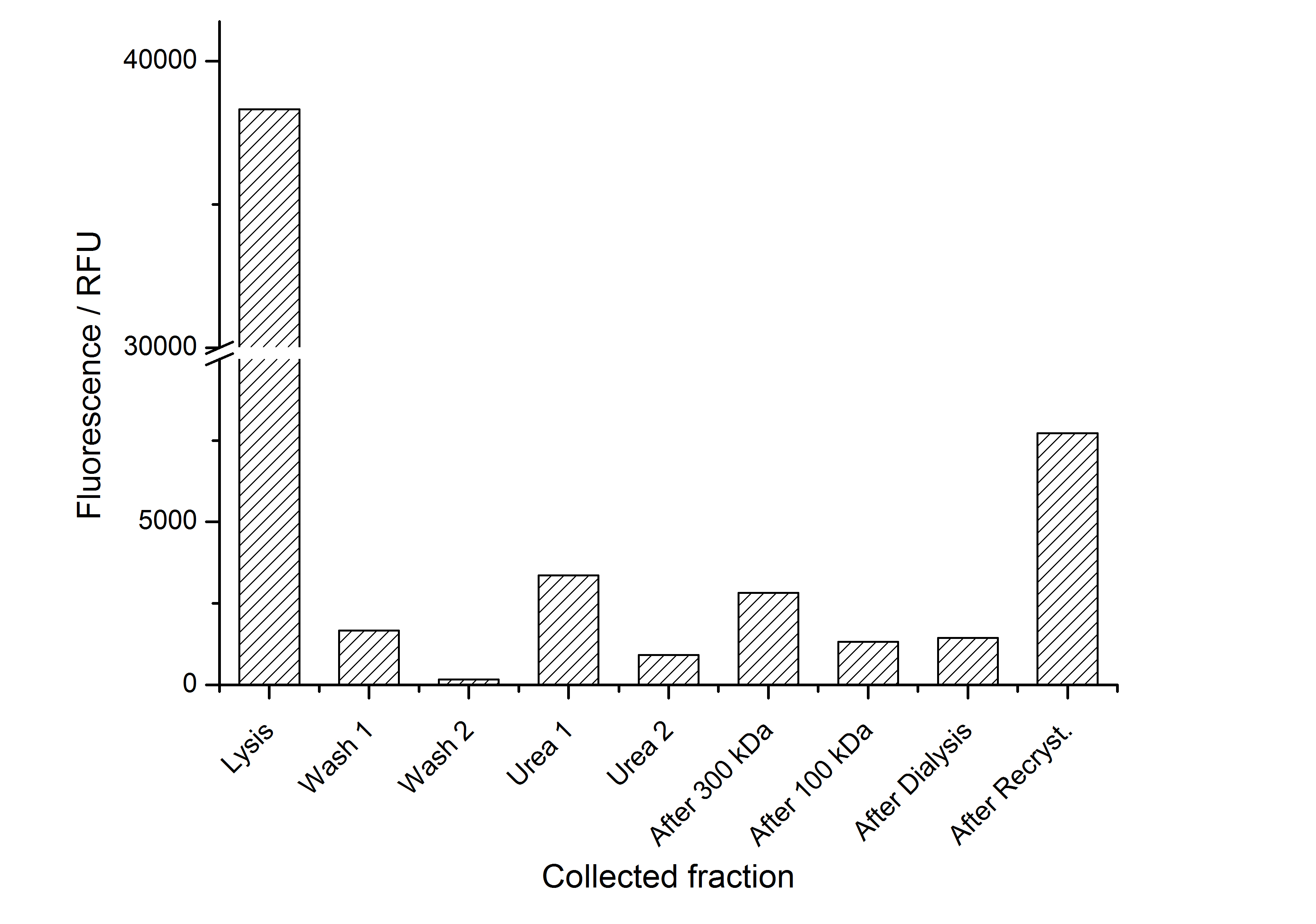
The fluorescence of the collected fractions of this purification strategy is shown in the following figure 3:
A lot of protein is lost during the purification especially after centrifugation steps. The fluorescence in the urea containing fractions is lowered due to denaturation of the fluorescent protein. Some fluorescence could be regenerated by the recrystallization in HBSS. This purification strategy is very simple and can be carried out by nearly everyone in any lab being one first step to enable real do it yourself nanobiotechnology.
Final purification strategy
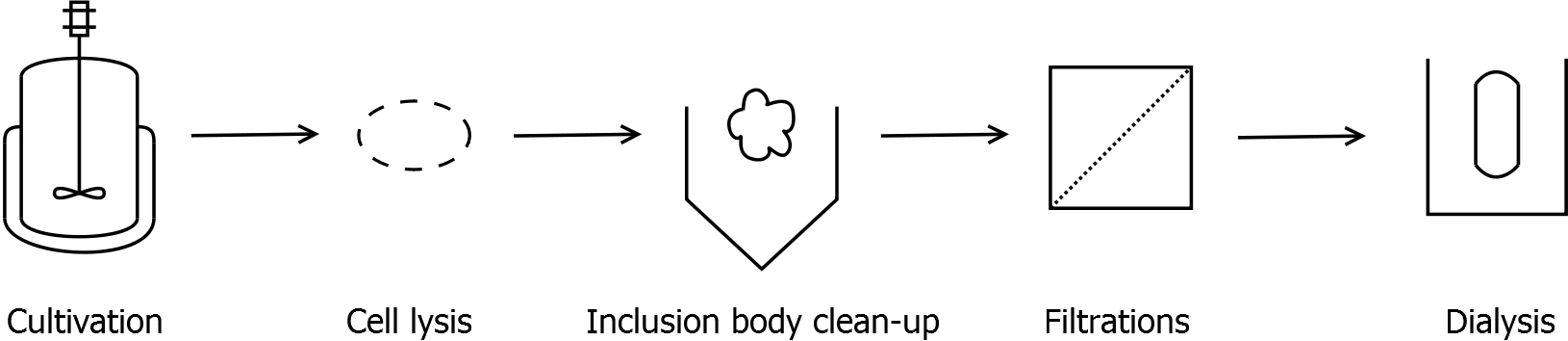
Scheme of purification strategy for SgsE (fusion) proteins:
First, SgsE is expressed in E. coli under the control of a T7 / lac promoter for separation of growth and production phase due to metabolic stress of the S-layer expression. Because the SgsE protein is forming inclusion bodies in E. coli, an inclusion body purification with urea follows the cell lysis. The S-layers are further concentrated and purified by two ultrafiltration / diafiltration steps (300 kDa and 100 kDa) and afterwards dialysed against water leading to the precipitation of water-insoluble proteins. The supernatant contains the monomeric SgsE solution.
Click for detailed information
Immobilization behaviour
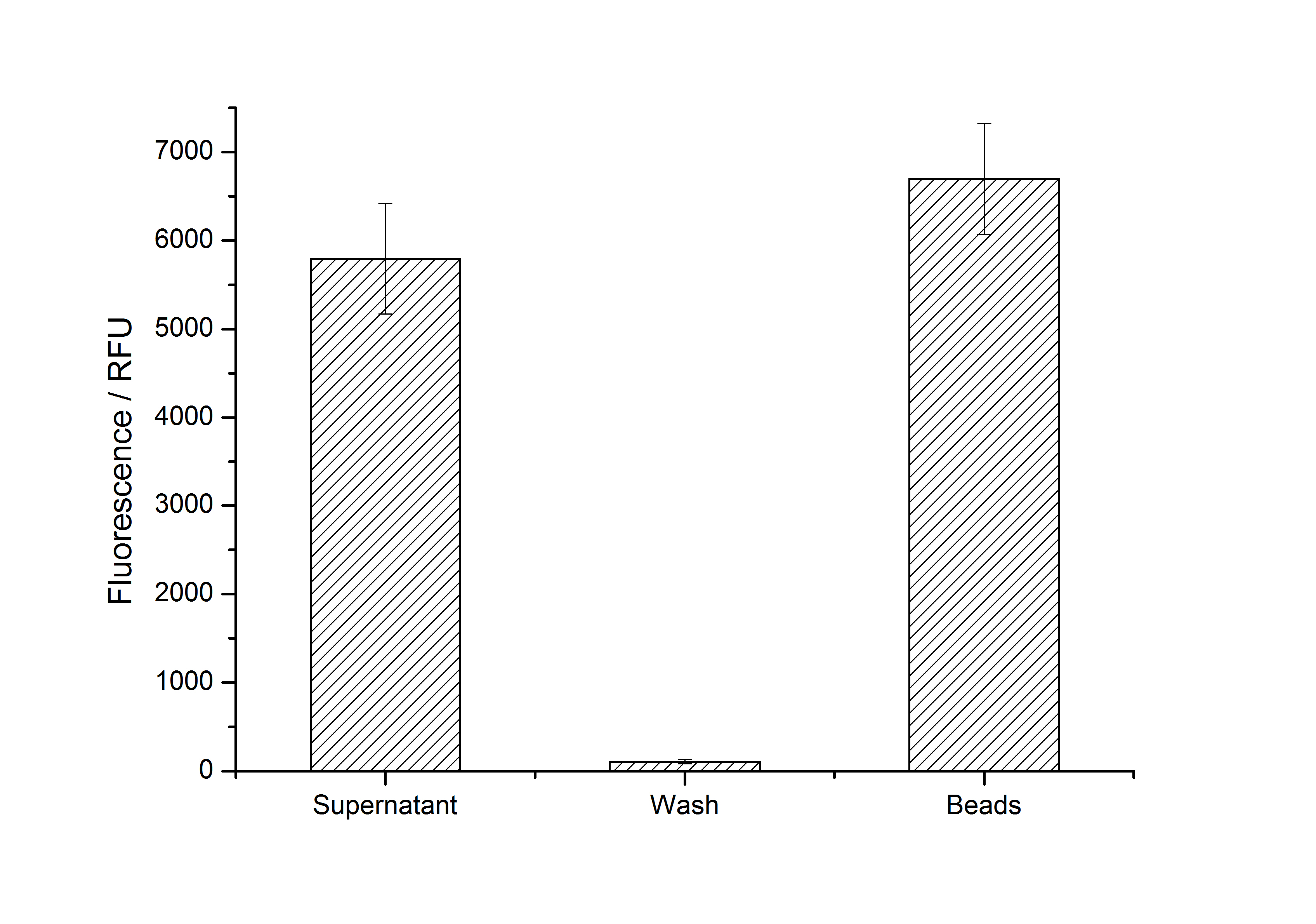
After purification, solutions of monomeric SgsE S-layer proteins can be recrystallized and immobilized on silicon dioxide beads in HBSS (Hank's buffered saline solution). After the recrystallization procedure the beads are washed with and stored in ddH2O at 4 °C in the dark. The fluorescence of the collected fractions of a recrystallization experiment with <partinfo>K525305</partinfo> are shown in fig. 4. 100 mg beads were coated with 100 µg of protein. The figure shows, that not all of the protein is immobilized on the beads (supernatant fraction) but the immobilization is pretty stable (very low fluorescence in the wash). After the immobilization, the beads show a high fluorescence indicating the binding of the SgsE | mCitrine fusion protein.
Optimal bead to protein ratio for immobilization
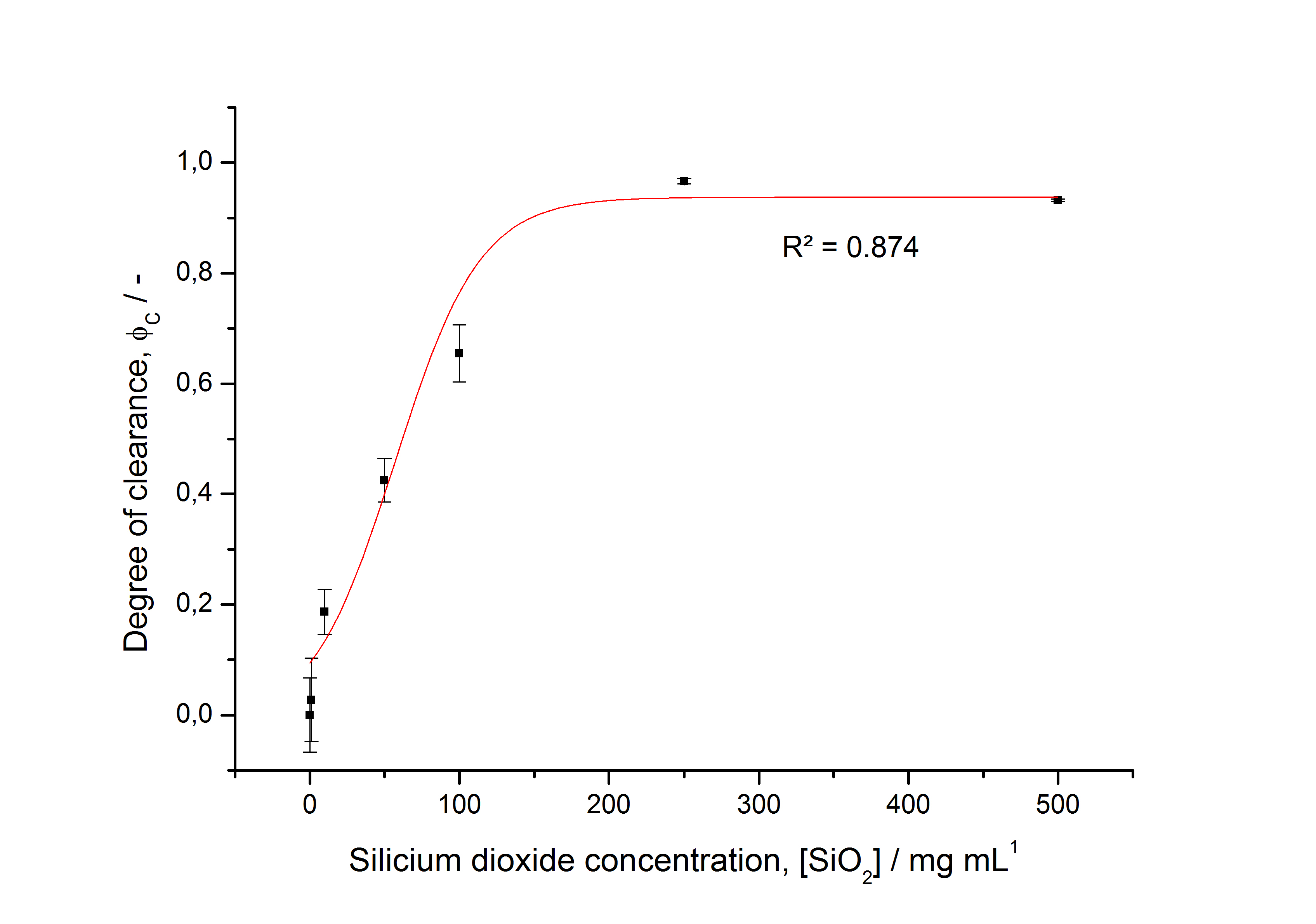
To determine the optimal ratio of silica beads to protein for immobilization, the degree of clearance ϕC in the supernatant is calculated and plotted against the concentration of silica beads used in the accordant immobilization experiment (compare fig. 5):
The data was collected in three independent experiments. The fluorescence of the samples was measured in the supernatant of the immobilization experiment after centrifugation of the silica beads. The fluorescence of the control was measured in a sample which was treated exactly like the others but no silica beads were added. 100 µg protein was used for one immobilization experiment. The data was fitted with a sigmoidal dose-response function of the form
with the Hill coefficient p, the bottom asymptote A1, the top asymptote A2 and the switch point log(x0) (R² = 0.874).
The fit indicates that a good silica concentration for 100 µg of protein is 150 - 200 mg mL-1. This set-up leads to saturated beads with low waste of protein. So a good protein / bead ratio to work with is 5 - 7 * 10-4.
 "
"