Team:Arizona State/Notebook/PCRLog
From 2011.igem.org
(Difference between revisions)
Ethan ward (Talk | contribs) |
|||
| (4 intermediate revisions not shown) | |||
| Line 39: | Line 39: | ||
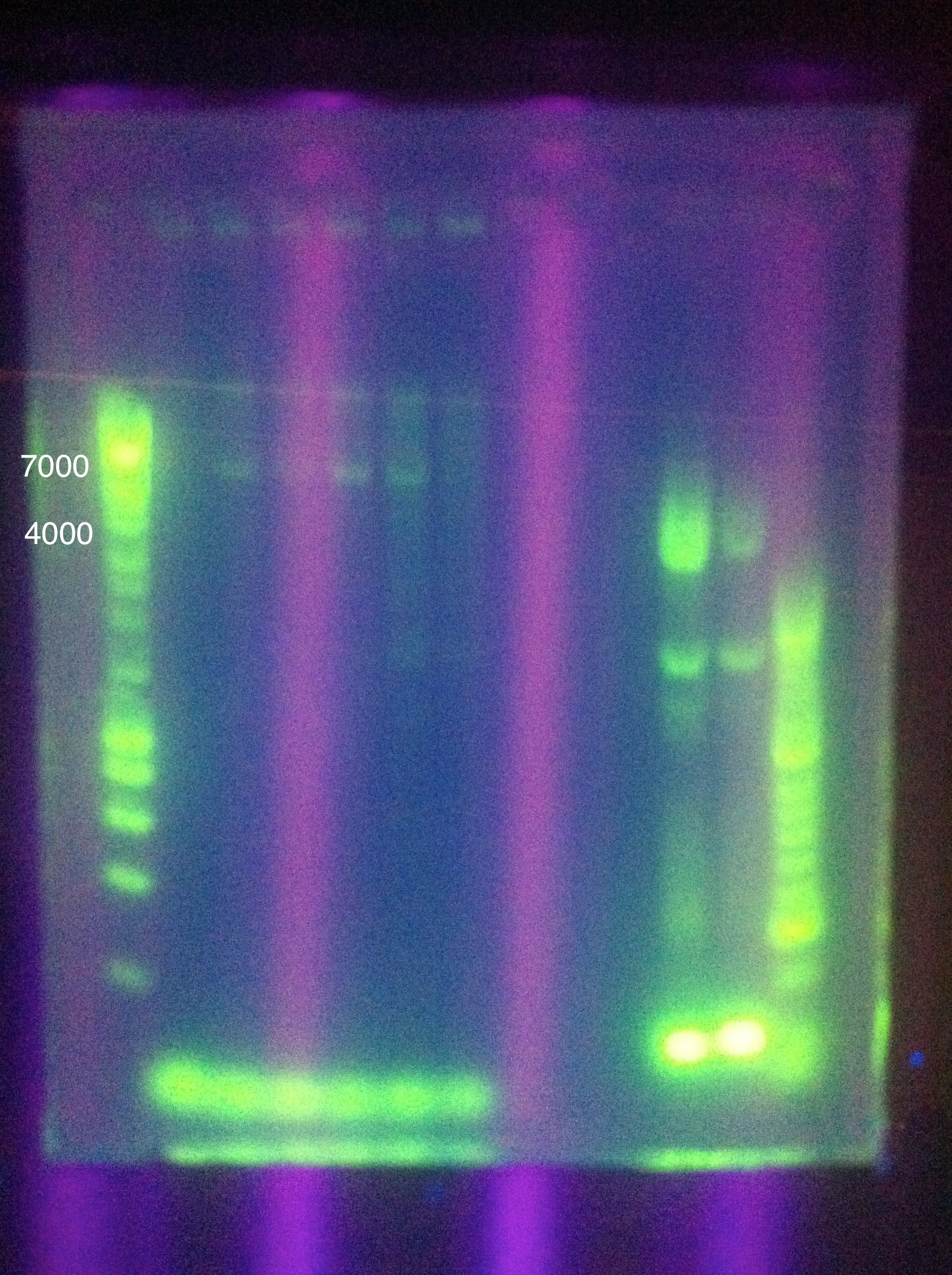
[[Image:ASU_720_CasABCDE.jpg|300px]] | [[Image:ASU_720_CasABCDE.jpg|300px]] | ||
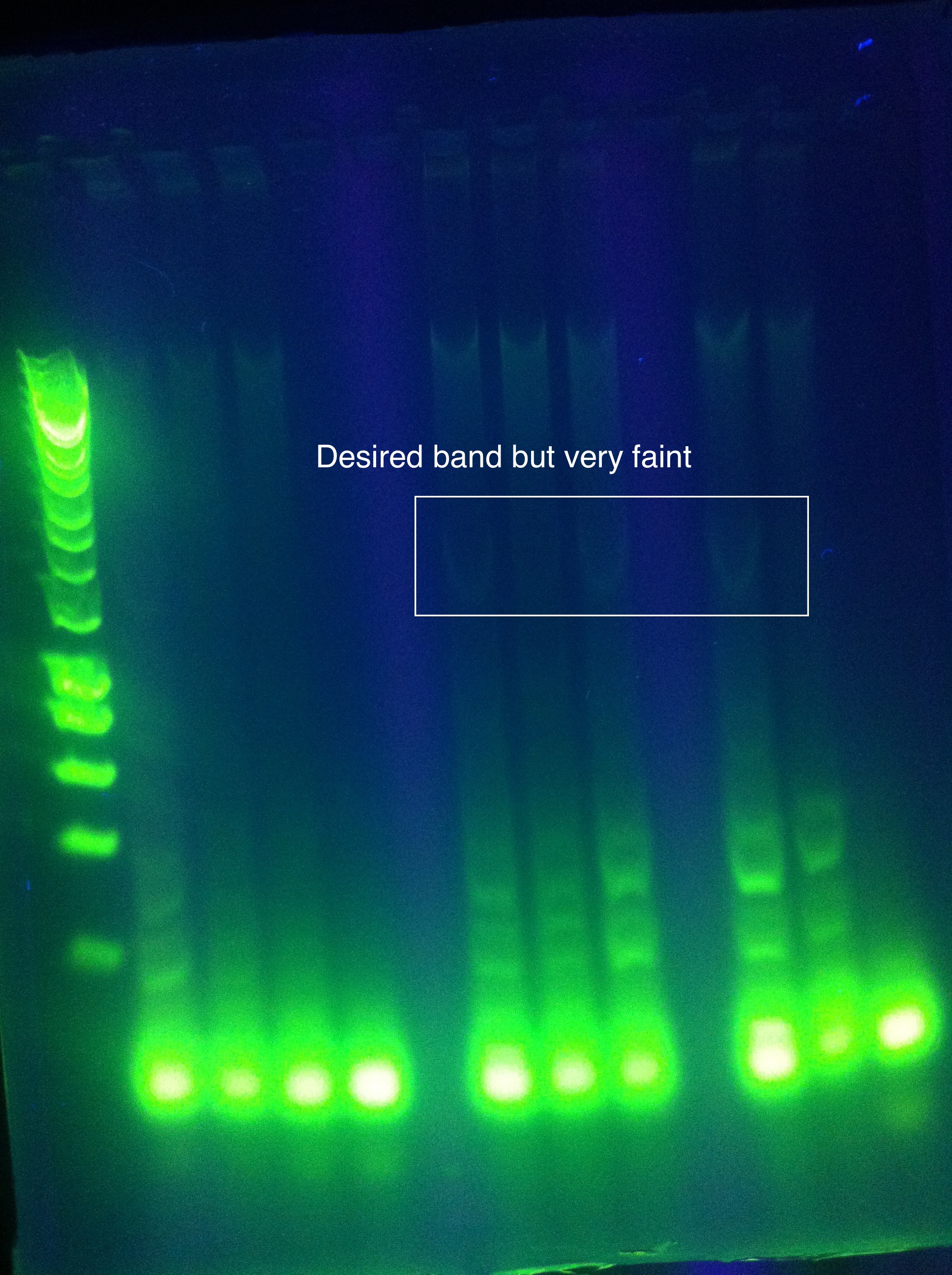
<br>CasABCDE: Nonspecific, nothing usable, no bands in target range, dimerization | <br>CasABCDE: Nonspecific, nothing usable, no bands in target range, dimerization | ||
| - | [[Image:ASU_720_Cas3.jpg|300px]] | + | <br>[[Image:ASU_720_Cas3.jpg|300px]] |
<br>Cas3: Small band at correct location, primer dimers | <br>Cas3: Small band at correct location, primer dimers | ||
| Line 47: | Line 47: | ||
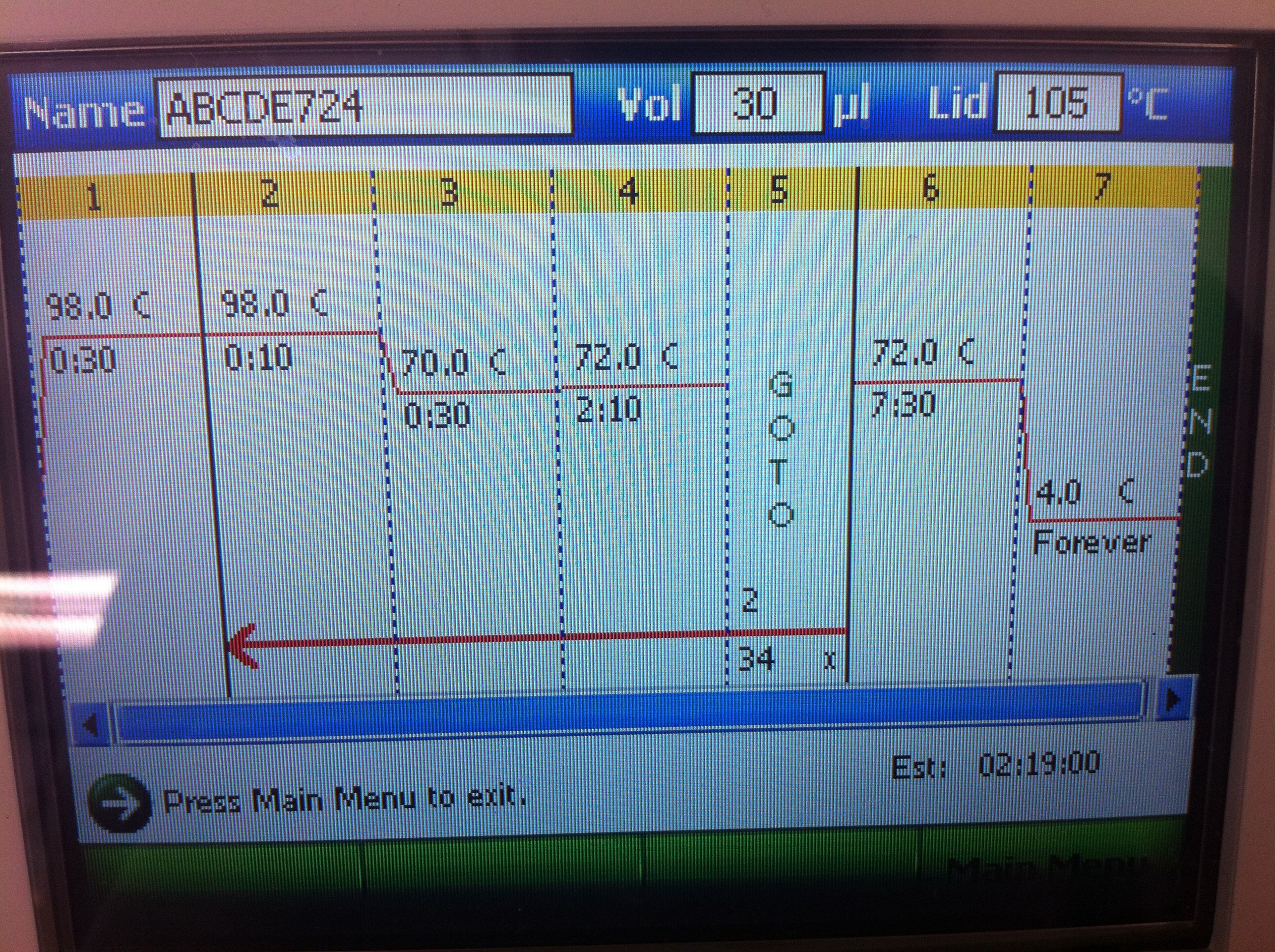
<b>CasABCDE Settings and Results</b>: | <b>CasABCDE Settings and Results</b>: | ||
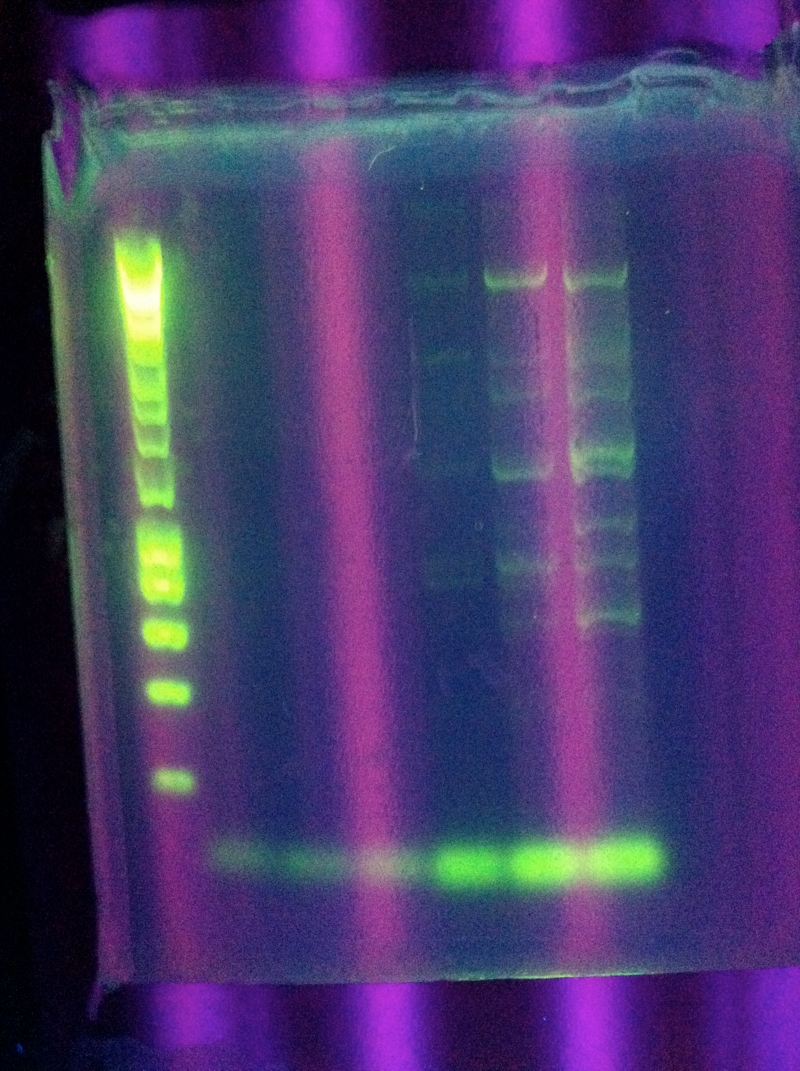
<br>[[Image:ASU_725_Settings_ABCDE.jpeg|300px]] | <br>[[Image:ASU_725_Settings_ABCDE.jpeg|300px]] | ||
| - | <br>[[Image:ASU_725_gel_abcde.jpeg|300px]] | + | <br><br>[[Image:ASU_725_gel_abcde.jpeg|300px]] |
<br>Nonspecific results, perhaps a hint of the correct bands in the second to last well | <br>Nonspecific results, perhaps a hint of the correct bands in the second to last well | ||
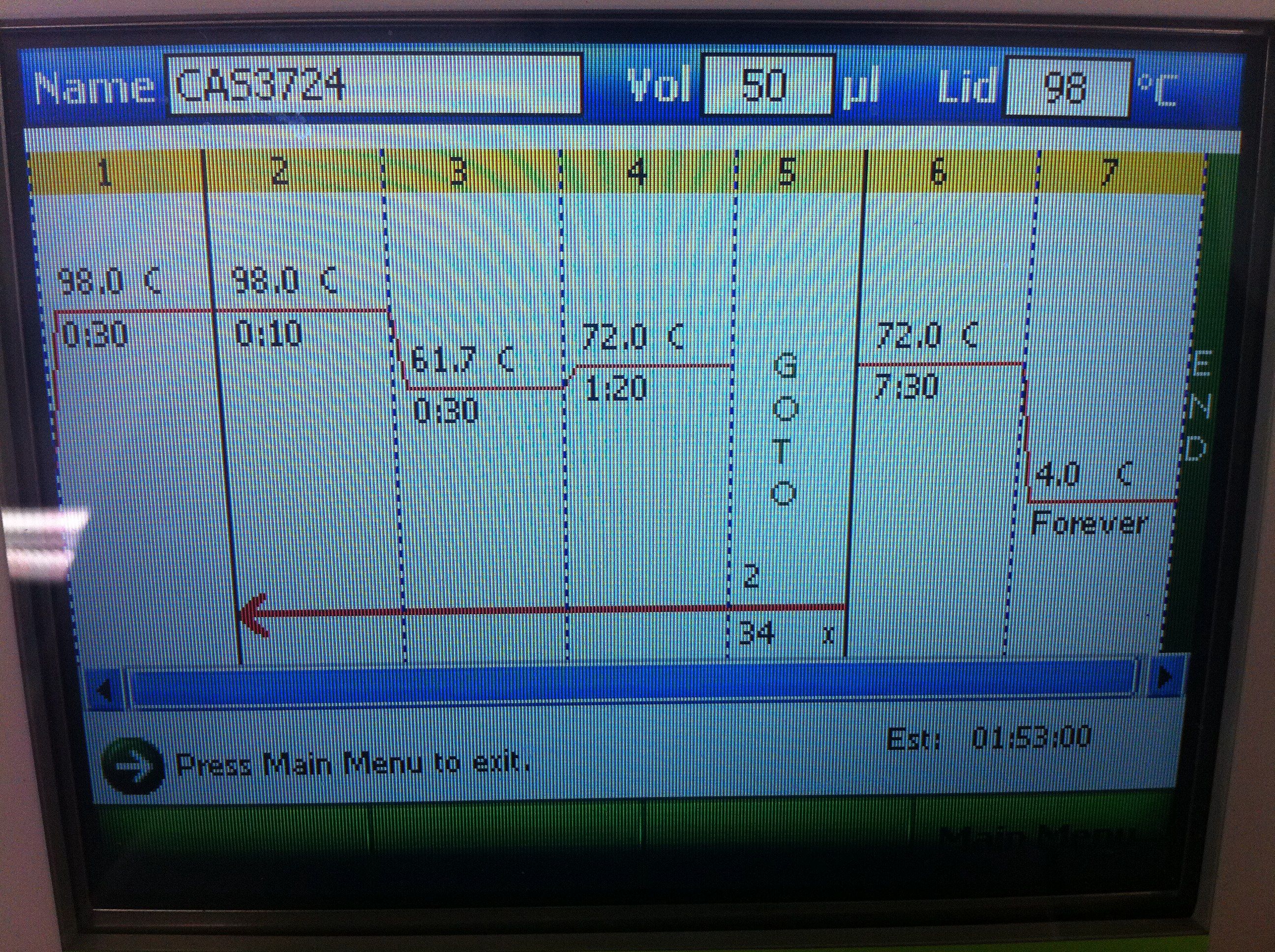
<br><b>Cas3 Settings and Results</b>: | <br><b>Cas3 Settings and Results</b>: | ||
<br>[[Image:ASU_725_Settings_Cas3.jpeg|300px]] | <br>[[Image:ASU_725_Settings_Cas3.jpeg|300px]] | ||
| - | <br>[[Image:ASU_725_gel_cas3.jpeg | + | <br><br>[[Image:ASU_725_gel_cas3.jpeg|300px]] |
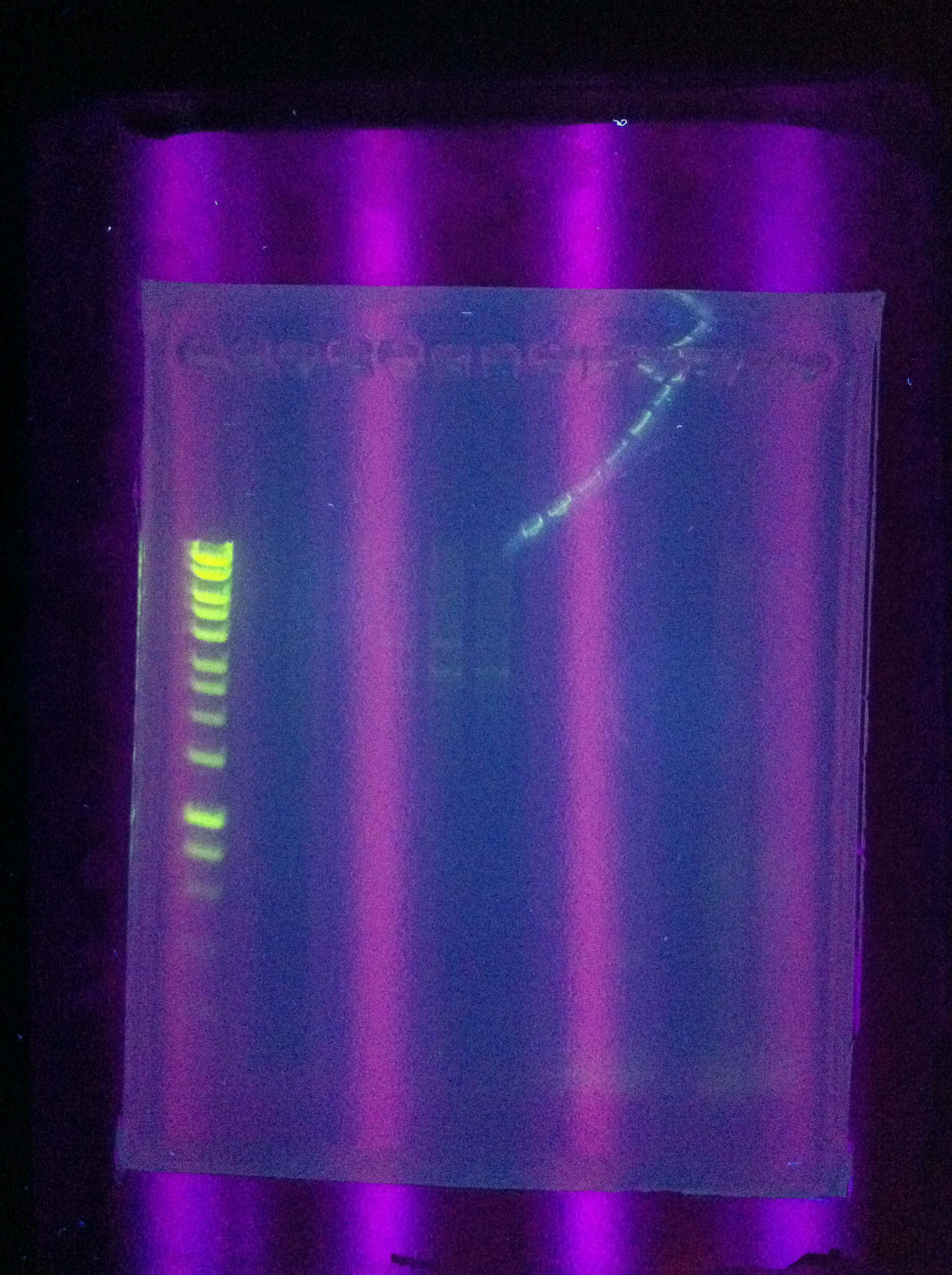
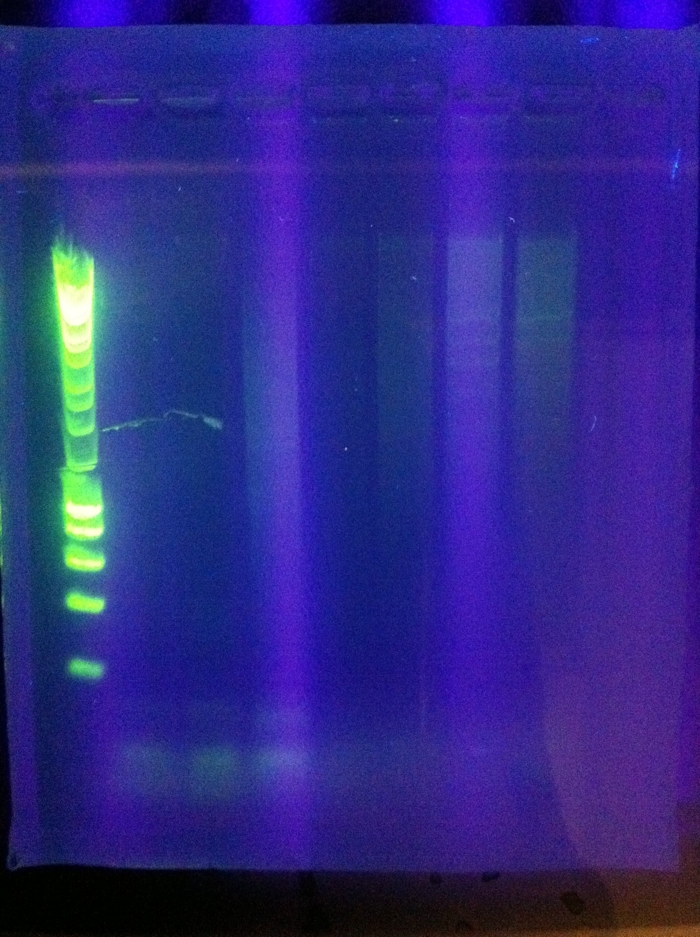
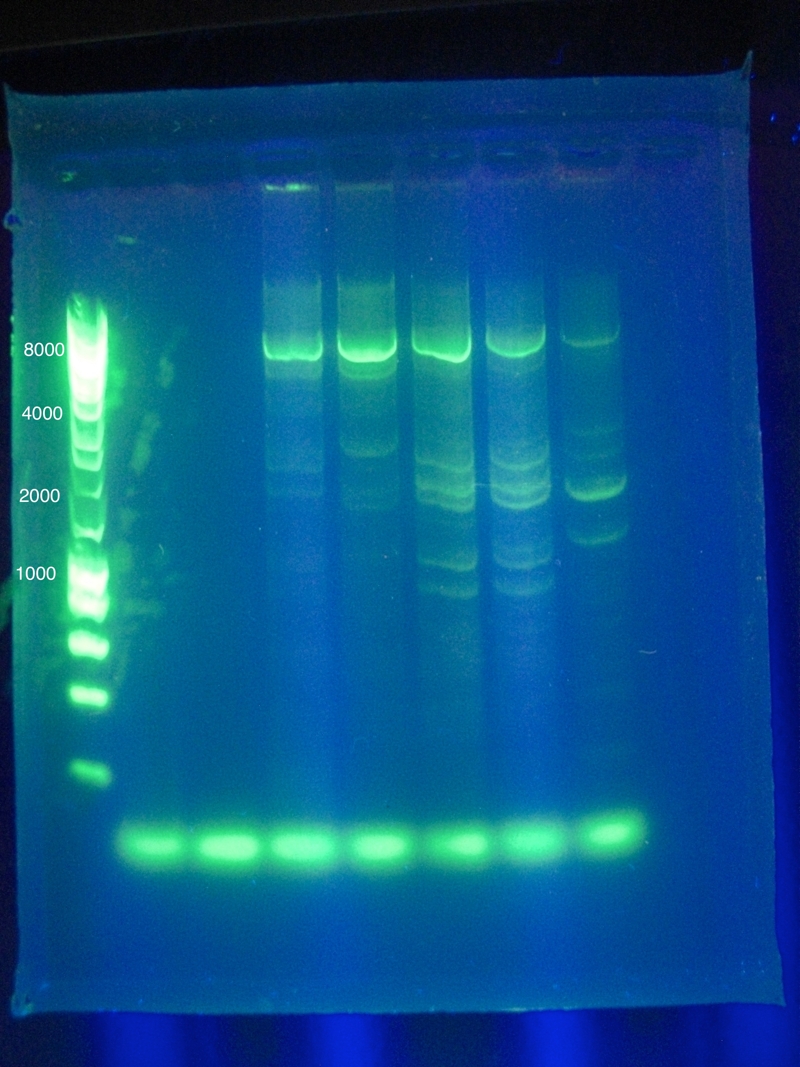
<br>Success for Cas3!! Extracted the band that lies around ~2500 (target is 2600). However, sequencing results were not conclusive. | <br>Success for Cas3!! Extracted the band that lies around ~2500 (target is 2600). However, sequencing results were not conclusive. | ||
| Line 60: | Line 60: | ||
<br><b>Cas3</b>: 62 degree annealing temp, 1:20 elongation | <br><b>Cas3</b>: 62 degree annealing temp, 1:20 elongation | ||
<p>Gel Results:</p> | <p>Gel Results:</p> | ||
| - | + | CasABCDE: mostly blank | |
| - | + | ||
<br>Cas3: broad nonspecific bands around desired area | <br>Cas3: broad nonspecific bands around desired area | ||
| Line 68: | Line 67: | ||
<br><b>CasABCDE (old)</b>: 63->66, three rows of 3 tubes | <br><b>CasABCDE (old)</b>: 63->66, three rows of 3 tubes | ||
<p>Gel Results:</p> | <p>Gel Results:</p> | ||
| - | [[Image:]] | + | [[Image:ASU_727_IMG_3952.jpg|300px]] |
<br>new: no good | <br>new: no good | ||
<br>old: mostly blank… not sure why this is. slight dimerization. | <br>old: mostly blank… not sure why this is. slight dimerization. | ||
| Line 76: | Line 75: | ||
<br><b>CasABCDE (new)</b>: TDWN728B, 72 to 65, -.2/cycle, 35 cycles, 2:10 elongation</p> | <br><b>CasABCDE (new)</b>: TDWN728B, 72 to 65, -.2/cycle, 35 cycles, 2:10 elongation</p> | ||
<p>Gel Results:</p> | <p>Gel Results:</p> | ||
| - | |||
<br>new: no good | <br>new: no good | ||
<br>old: no good | <br>old: no good | ||
| Line 83: | Line 81: | ||
<b>CasABCDE</b>: 68 Touchdown from 72 to 65, -.2/cycle, 35 cycles, 2:10 elongation | <b>CasABCDE</b>: 68 Touchdown from 72 to 65, -.2/cycle, 35 cycles, 2:10 elongation | ||
<p>Gel Results:</p> | <p>Gel Results:</p> | ||
| - | [[Image:]] | + | [[Image:ASU_729_PCR.jpg|300px]] |
<br>No good | <br>No good | ||
| Line 89: | Line 87: | ||
<b>Cas3</b>: Ran Cas3724 protocol | <b>Cas3</b>: Ran Cas3724 protocol | ||
<p>Gel Results:</p> | <p>Gel Results:</p> | ||
| - | [[Image:]] | + | [[Image: ASU_89_CasABCDE.jpg|300px]] |
<br>new: no good | <br>new: no good | ||
| Line 95: | Line 93: | ||
===August 4, 2011=== | ===August 4, 2011=== | ||
<p><b>Settings</b>: 98 initial denaturation for 30 seconds, Cycle (10sec at 98deg, anneal 30 sec at 63 deg, elongate 130sec at 72deg), 35 Cycles, Extension for 450 seconds</p> | <p><b>Settings</b>: 98 initial denaturation for 30 seconds, Cycle (10sec at 98deg, anneal 30 sec at 63 deg, elongate 130sec at 72deg), 35 Cycles, Extension for 450 seconds</p> | ||
| - | [[Image:]] | + | [[Image:ASU_PCR_Gel_84.jpg|300px]] |
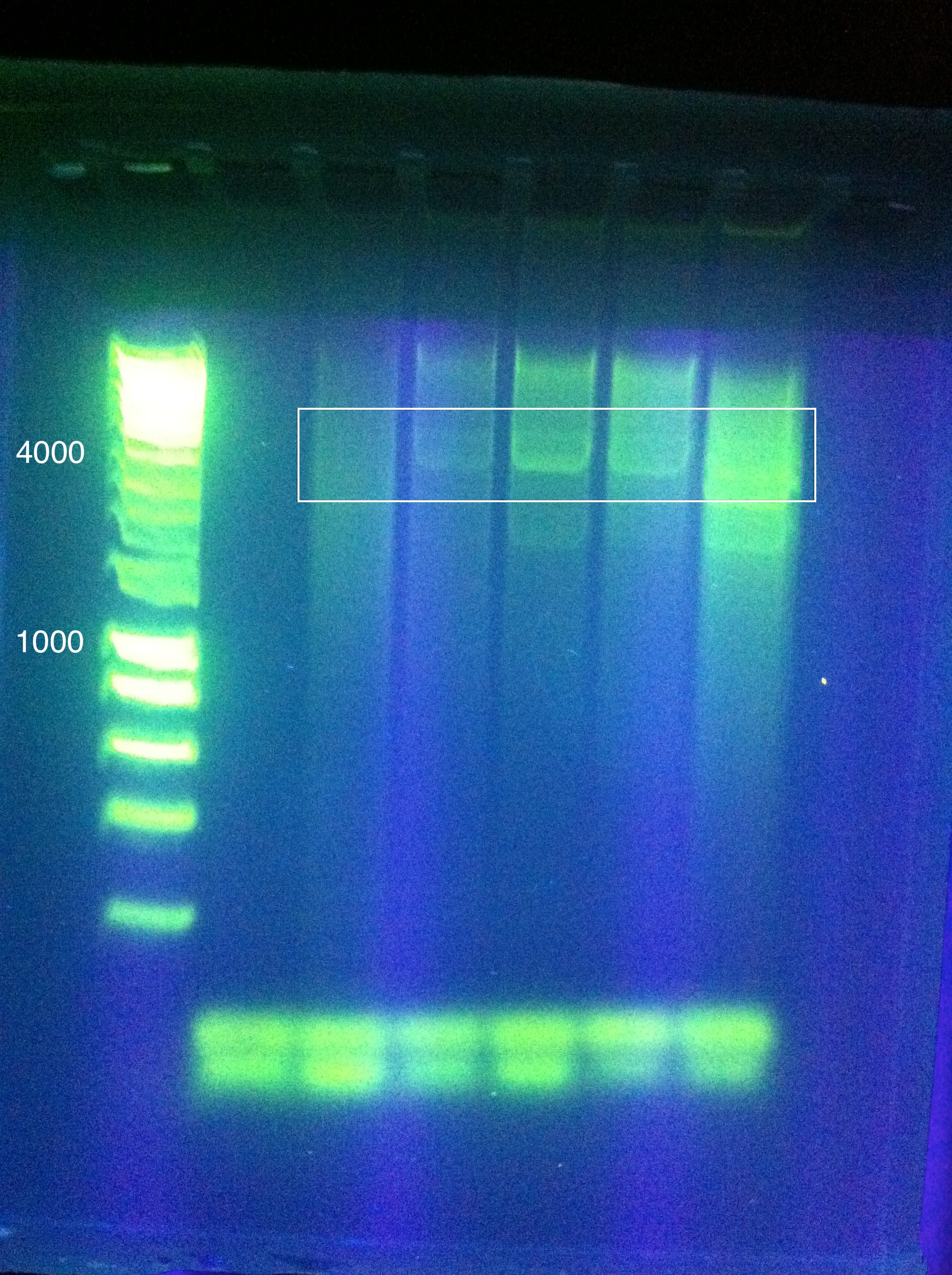
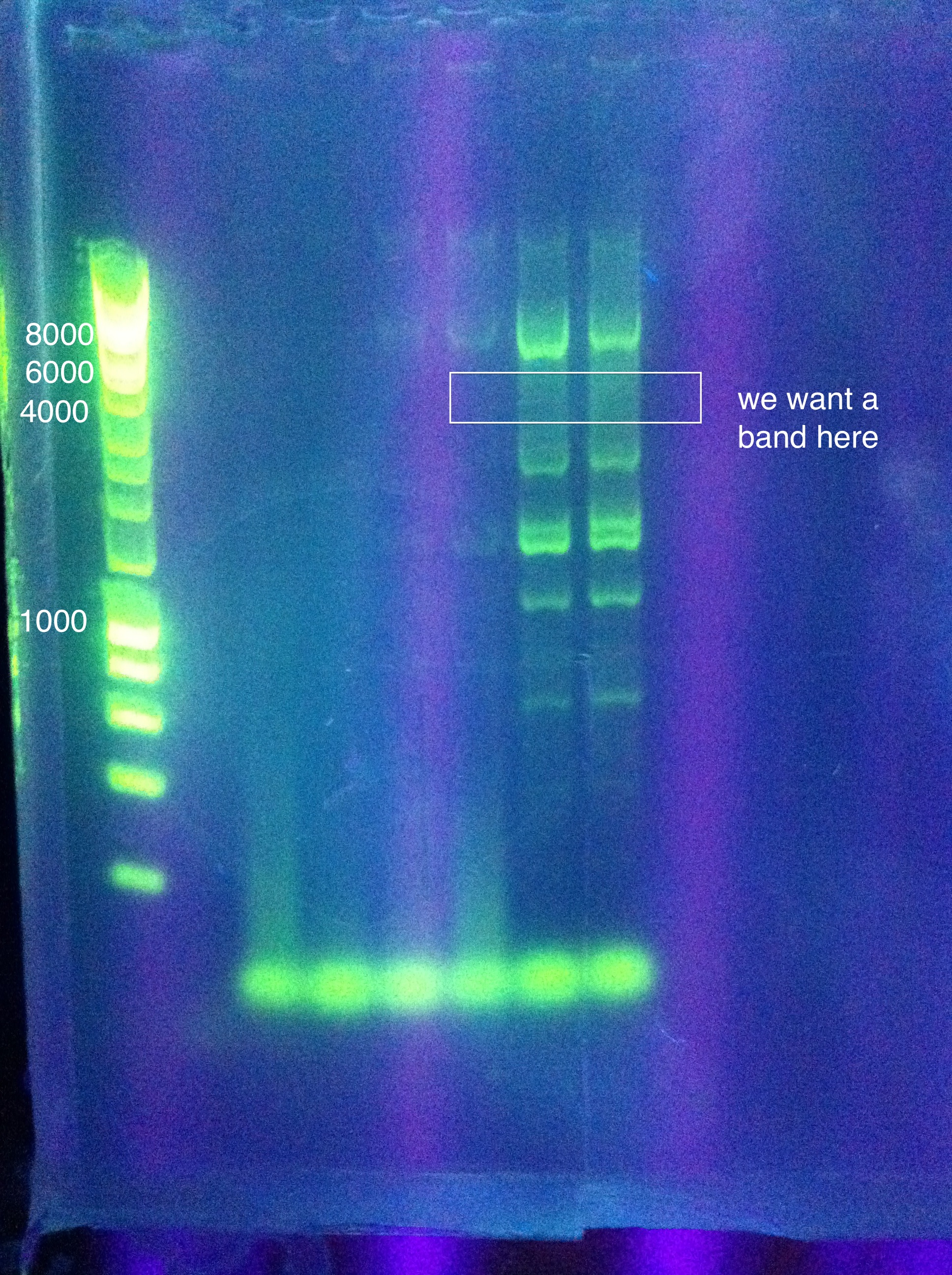
<br>Results show a band above where we think it ought to be (6 to 8 kbp instead of 4.3) | <br>Results show a band above where we think it ought to be (6 to 8 kbp instead of 4.3) | ||
However, this was extracted and an attempt at a nested PCR was made using the same settings to try and amplify this band even further. The results were blank. | However, this was extracted and an attempt at a nested PCR was made using the same settings to try and amplify this band even further. The results were blank. | ||
| Line 101: | Line 99: | ||
===August 8, 2011=== | ===August 8, 2011=== | ||
<p><b>Settings</b>:ABCDE808, which is same as 8/4 run but only 29 cycles in the hope that the bright band from the previous try would be the only one visible.</p> | <p><b>Settings</b>:ABCDE808, which is same as 8/4 run but only 29 cycles in the hope that the bright band from the previous try would be the only one visible.</p> | ||
| - | [[Image:]] | + | [[Image:ASU_88_PCR.jpg|300px]] |
<br>Looks very similar to previous results. Again, a band above where we would like to see it. | <br>Looks very similar to previous results. Again, a band above where we would like to see it. | ||
===August 8, 2011=== | ===August 8, 2011=== | ||
<p><b>Settings</b>: Retry same protocol in thermocycler, however increase back to 35 cycles.</p> | <p><b>Settings</b>: Retry same protocol in thermocycler, however increase back to 35 cycles.</p> | ||
| - | [[Image:]] | + | [[Image: ASU_810_Cas3_1.jpg|300px]] |
<br>Not as good as the original run, though there may be faint bands where we would like to see them. For some reason the brightest band is again somewhere between 6k and 8k (though it's a bit hard to tell because the ladder curves annoyingly at the top), instead of the desired 4300. However, we have ordered the nested PCR primers, so perhaps that will offer a better solution to getting Cas ABCDE. In the meantime, we'll focus again on Cas3 and try to extract that and get a good sequencing result so we can say definitively that we have it. | <br>Not as good as the original run, though there may be faint bands where we would like to see them. For some reason the brightest band is again somewhere between 6k and 8k (though it's a bit hard to tell because the ladder curves annoyingly at the top), instead of the desired 4300. However, we have ordered the nested PCR primers, so perhaps that will offer a better solution to getting Cas ABCDE. In the meantime, we'll focus again on Cas3 and try to extract that and get a good sequencing result so we can say definitively that we have it. | ||
===August 10, 2011 (Evening)=== | ===August 10, 2011 (Evening)=== | ||
<p><b>Settings</b>: Ran another PCR for ABCDE, same settings as before but one degree higher for annealing temp. Also added DMSO.</p> | <p><b>Settings</b>: Ran another PCR for ABCDE, same settings as before but one degree higher for annealing temp. Also added DMSO.</p> | ||
| - | [[Image:]] | + | [[Image: ASU_810_Cas3_2.jpg|300px]] |
<br>Similar result, less bands from DMSO but consequently much fainter bands. Dimerization still evident. | <br>Similar result, less bands from DMSO but consequently much fainter bands. Dimerization still evident. | ||
| Line 118: | Line 116: | ||
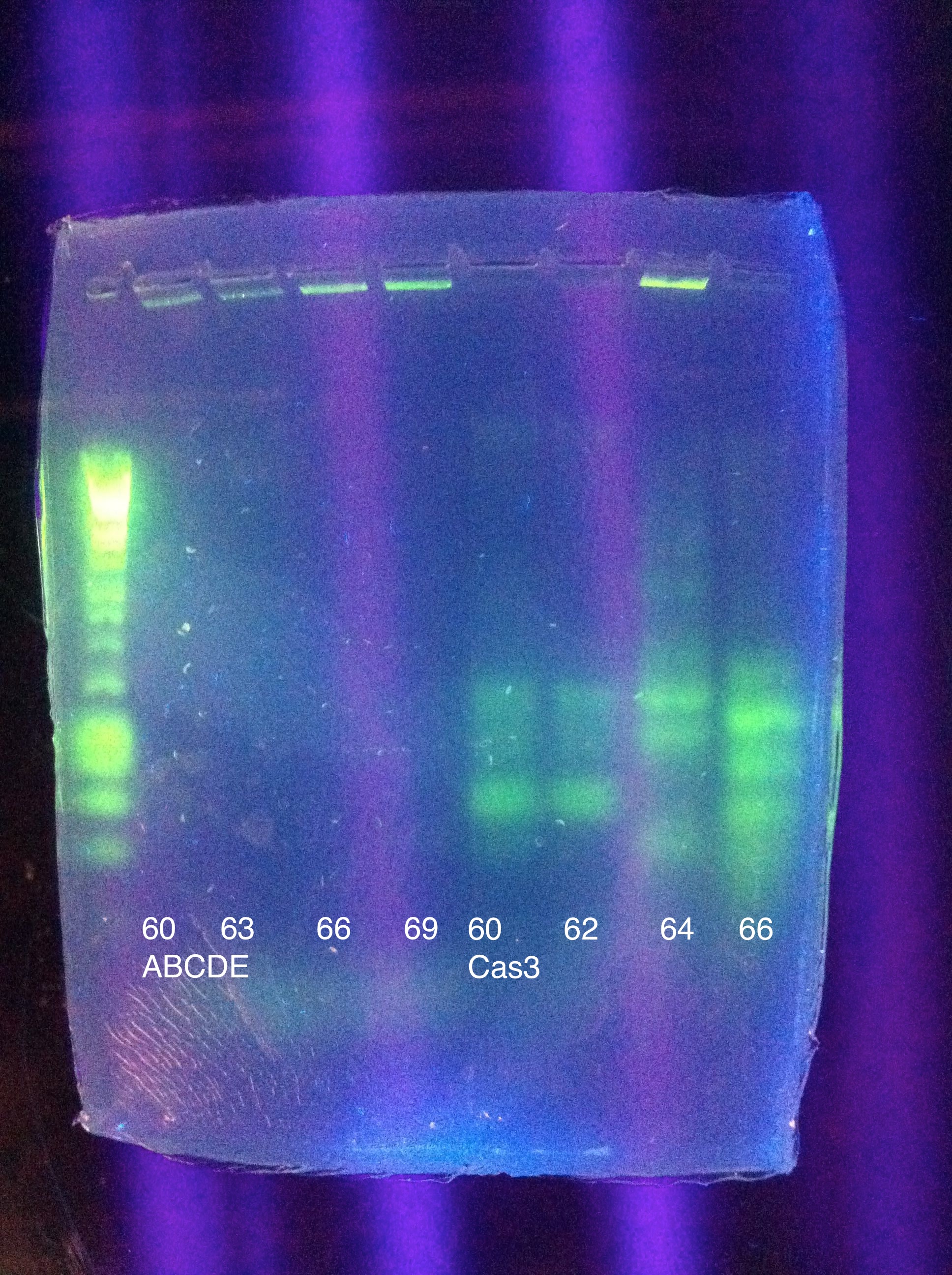
<b>CasABCDE</b>: MgCl2 --> 2uL, 4uL, 5uL, Temp --> 60, 63, 66, 69 | <b>CasABCDE</b>: MgCl2 --> 2uL, 4uL, 5uL, Temp --> 60, 63, 66, 69 | ||
<br><b>Cas3</b>: MgCl2 --> 2uL, 4uL, 5uL, Temp --> 60, 62, 64, 66 | <br><b>Cas3</b>: MgCl2 --> 2uL, 4uL, 5uL, Temp --> 60, 62, 64, 66 | ||
| - | [[Image:]] | + | <br>[[Image: ASU_ABCDE_Pranqster.jpeg|300px]] |
<br>CasABCDE: No bands | <br>CasABCDE: No bands | ||
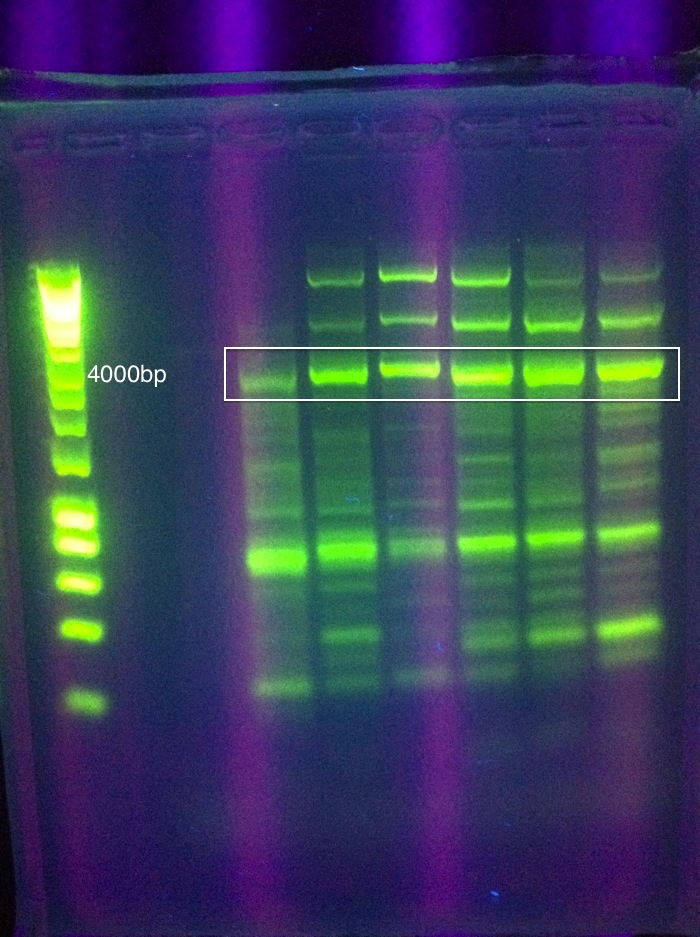
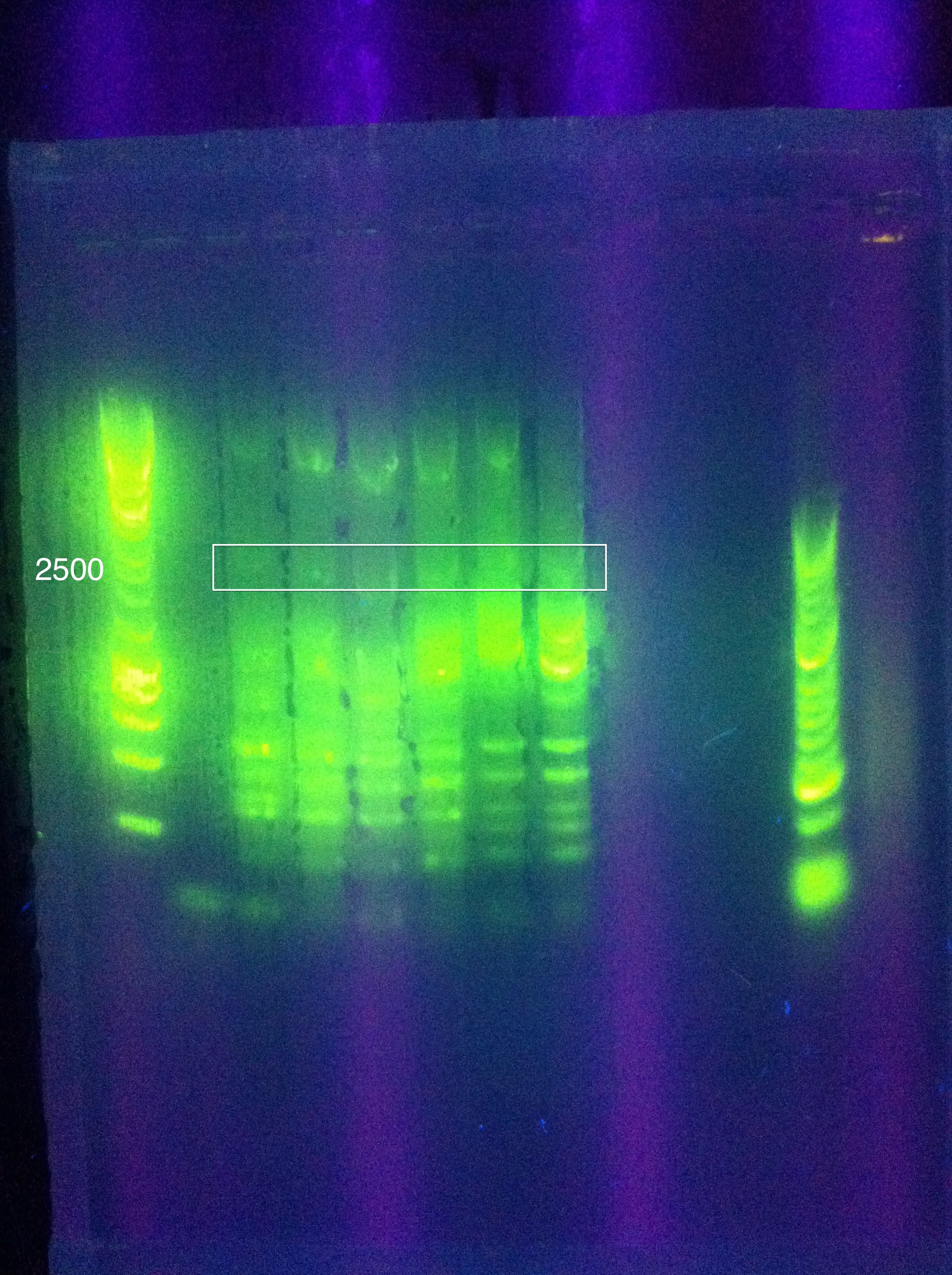
| + | <br>[[Image: ASU_811_Gradient_gel_result.jpeg|300px]] | ||
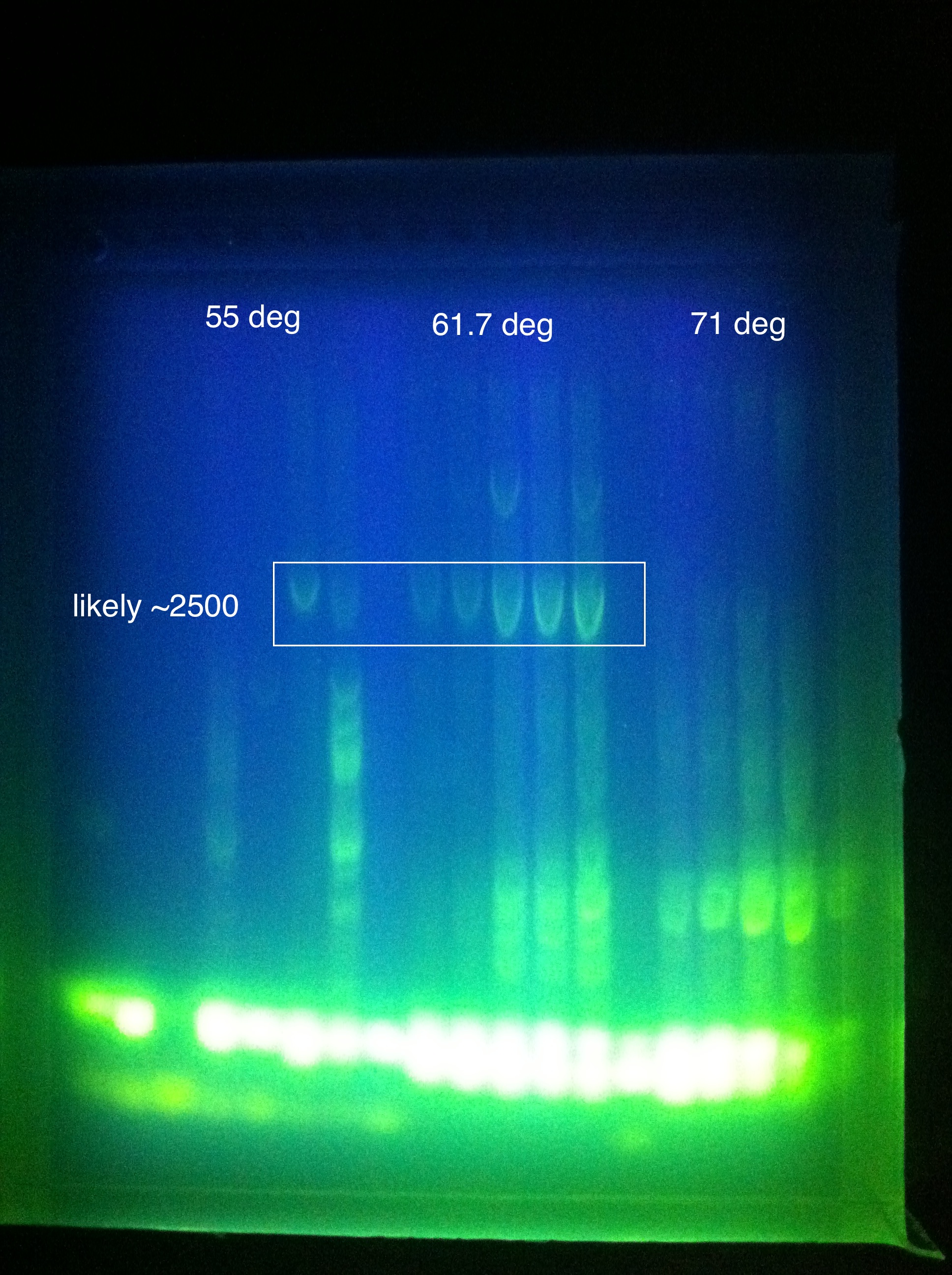
<br>Cas3: 60 and 62 have bands less than our desired target, up to perhaps 1500; 64 had a couple of bands above this, perhaps one faint one near our 2600 target; 66 had more nonspecific amplification, and no clear bands in our target range | <br>Cas3: 60 and 62 have bands less than our desired target, up to perhaps 1500; 64 had a couple of bands above this, perhaps one faint one near our 2600 target; 66 had more nonspecific amplification, and no clear bands in our target range | ||
<p>Moving forward to nested PCR for ABCDE, we will see how this goes. Cas3 we'll have to keep trying…perhaps it may be worth it to try DMSO, longer elongation time, something. Maybe Taq.</p> | <p>Moving forward to nested PCR for ABCDE, we will see how this goes. Cas3 we'll have to keep trying…perhaps it may be worth it to try DMSO, longer elongation time, something. Maybe Taq.</p> | ||
| Line 125: | Line 124: | ||
===August 12, 2011=== | ===August 12, 2011=== | ||
*PCR using Nest primers for ABCDE | *PCR using Nest primers for ABCDE | ||
| - | + | :*Tm1: 74, Tm2: 77 --> Anneal at 72 deg (higher than this is not recommended) | |
| - | + | :*Used 2-step Phusion protocol | |
| - | + | ::*98 deg for 30 sec | |
| - | + | ::*{98deg for 10 sec, 72 deg for 2:30} X 35 cycles | |
| - | + | ::*Extension for 7:30 at 72 deg | |
| - | + | ::*Held at 4deg until run was cancelled | |
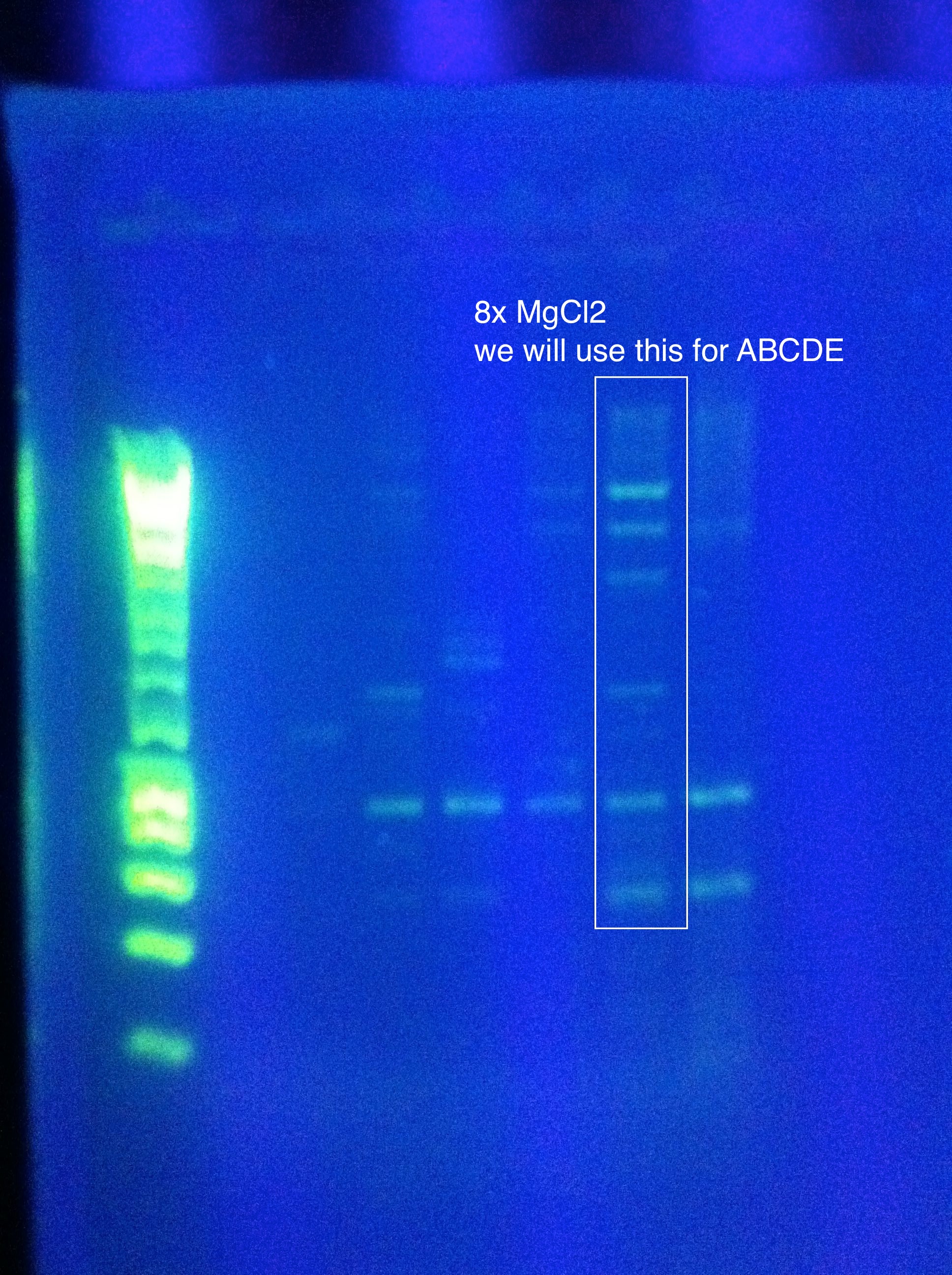
<br>Gel Results: | <br>Gel Results: | ||
| - | [[Image:]] | + | <br>[[Image:ASU_812_Nest.jpeg|300px]] |
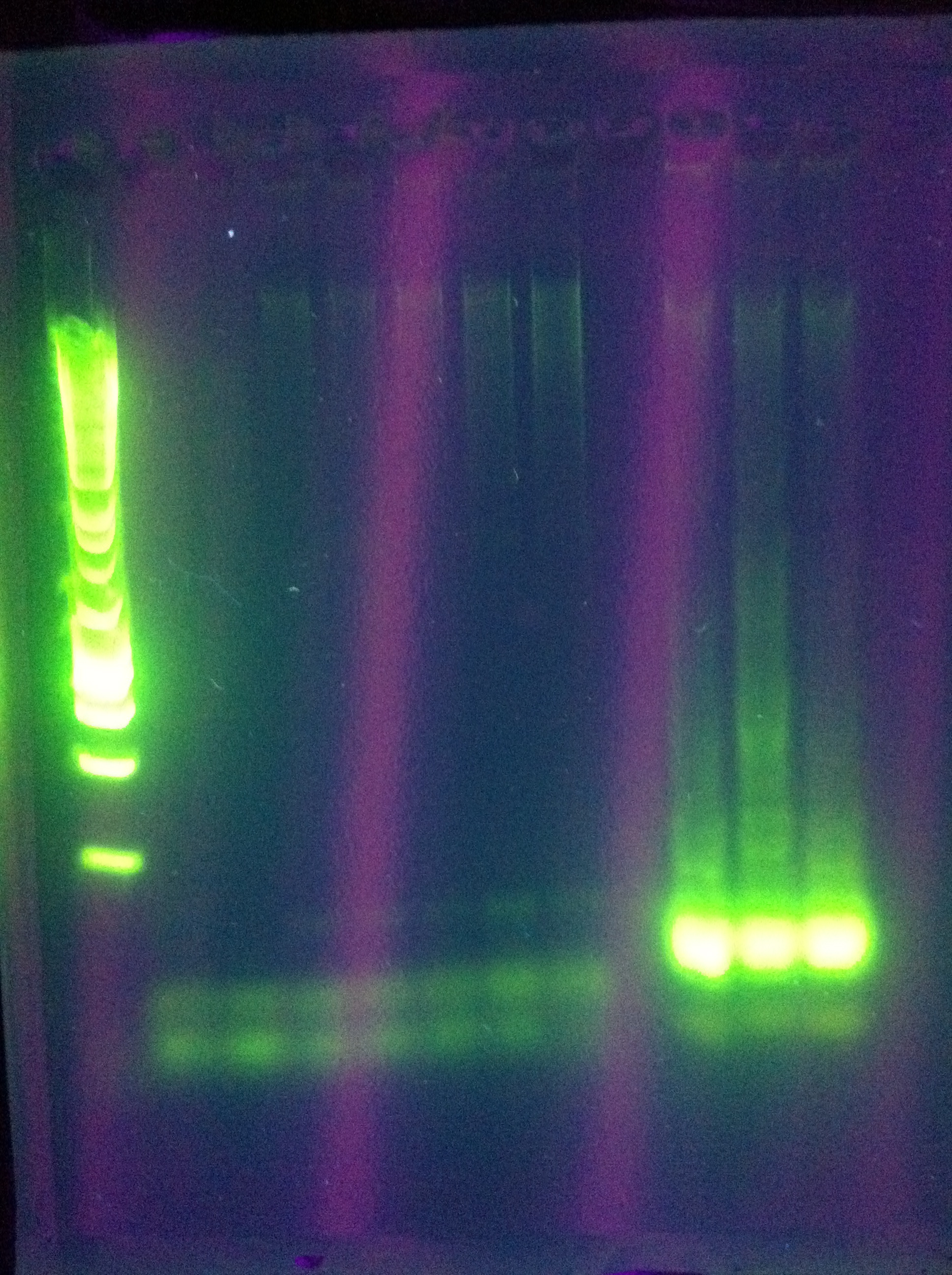
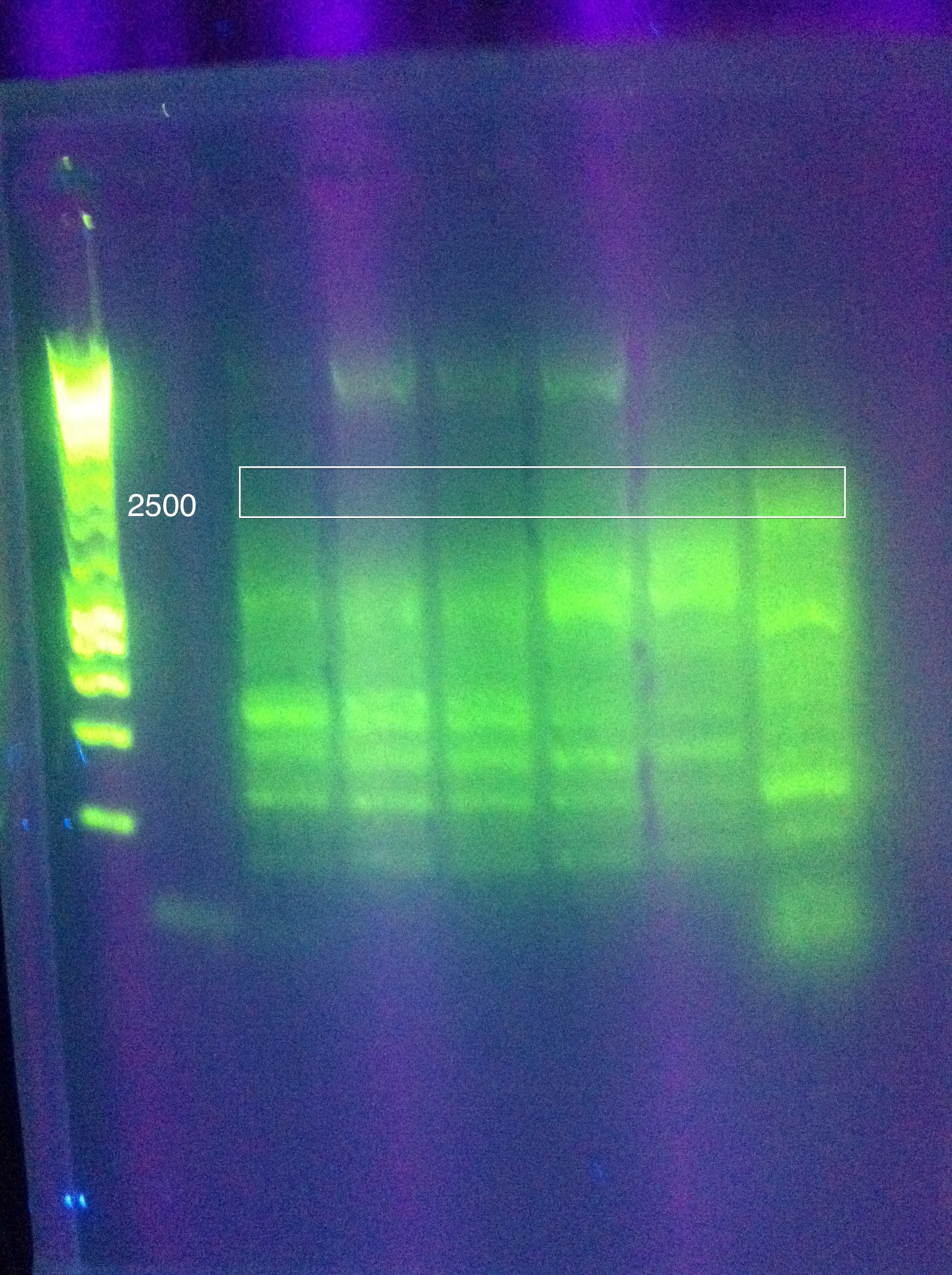
<br>The 8x MgCl2 tube has the best result | <br>The 8x MgCl2 tube has the best result | ||
<br>Nice clear bands at around 600, 1k, 2k, 4k, 6k, 8k | <br>Nice clear bands at around 600, 1k, 2k, 4k, 6k, 8k | ||
<br>PLAN: use this as template for ABCDE PCR | <br>PLAN: use this as template for ABCDE PCR | ||
<br>Use 1uL per tube (note, we don't know the concentration of the strand we want as template, so this is our best guess as to what would get us between 1pg and 10ng as recommended by the Phusion protocol for non-genomic DNA) | <br>Use 1uL per tube (note, we don't know the concentration of the strand we want as template, so this is our best guess as to what would get us between 1pg and 10ng as recommended by the Phusion protocol for non-genomic DNA) | ||
| - | |||
| - | |||
| - | |||
| - | |||
}} <!-- close content attribute for menubar --> | }} <!-- close content attribute for menubar --> | ||
Latest revision as of 04:31, 29 September 2011
 "
"