Team:ETH Zurich/Biology/MolecularMechanism
From 2011.igem.org
(→Plasmid strategy) |
(→Plasmid strategy) |
||
| Line 38: | Line 38: | ||
==Plasmid strategy == | ==Plasmid strategy == | ||
| - | To variate the copy number of the different components of our system, we introduced 3 plasmid with different origin of replication in our system. One with | + | To variate the copy number of the different components of our system, we introduced 3 plasmid with different origin of replication in our system. One with pBR322 for high copy which has about 15-20 copies per cell, one p15A for medium copy with 10-12 copies per cell and a pSC101 for low copy numbers with 5 copies per cell. |
| + | |||
| + | {| border="1" align="center" style="text-align:left;" | ||
| + | |Origin | ||
| + | |Genes | ||
| + | |- | ||
| + | |pBR322 | ||
| + | |P<sub>tet</sub>-LacI<sub>M1</sub>; P<sub>lac</sub>-GFP<sub>LVA</sub>; P<sub>luxR</sub>-RFP | ||
| + | |- | ||
| + | |p15A | ||
| + | |P<sub>U</sub>-CI; λ<sub>P</sub>-LacI; P<sub>const</sub>-luxR | ||
| + | |- | ||
| + | |psC101 | ||
| + | |P<sub>const</sub>-XylR; degadation pathway | ||
| + | |} | ||
| + | |||
http://www.springerlink.com/content/3fj1xxl9p42kx8l5/ | http://www.springerlink.com/content/3fj1xxl9p42kx8l5/ | ||
Revision as of 14:05, 20 September 2011
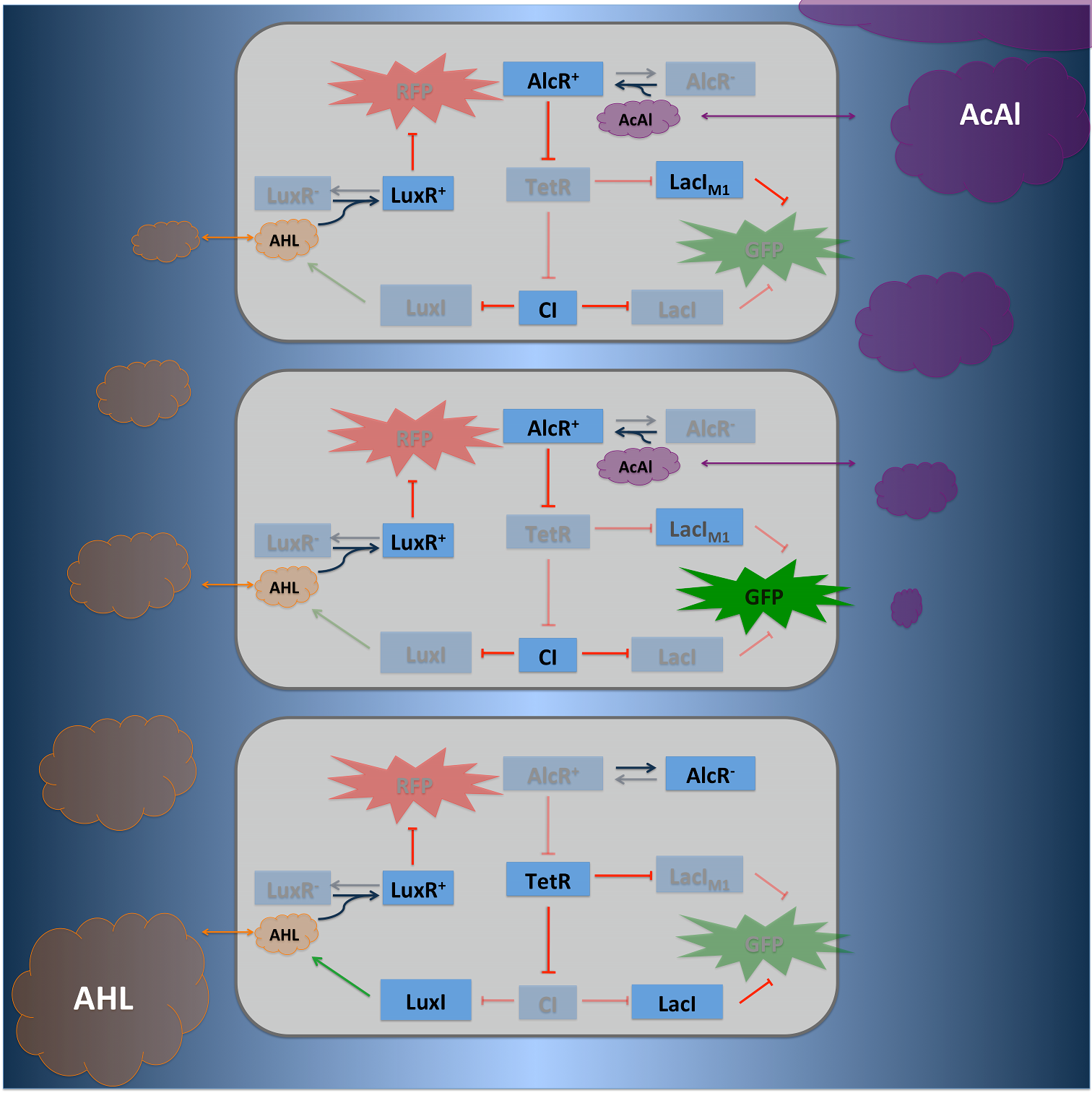
| Circuit design |
| ||
| We combined a Smoke-sensitive band-pass filter with GFP output with a quorum-sensing diffusive mechanism that alarms the user of the system to high Xylene levels by expressing RFP. | |||
Circuit designSmoColi is a bacterio quantifier which can be activated with different signal, we implemented two of them acetaldehyde and xylene. The concentration gradient which is needed for SmoColi is either naturally or achieved by synthetic cellular degradation. For example in case of Xylene we included the upper Tol pathway of Pseudomonas putida in SmoColi [1]. In contrast acetaldeydhde is naturally degraded. In our system we can use different small molceules as an activating input for the bandpass-filter [2]. The repressor which is activated by the small molecule is constantly expressed and enhances the expression of the LacIM1 repressor (codon-modified LacI) and the lambda repressor CI, which are under control of the Xyl-Promotor, respectively. High xylene concentration results in high cytoplasmic levels of CI and of LacIM1 and repression of the green fluorescent protein (GFP). Cells that are far from the input have low xylene concentrations, because of xylene degradation. Accordingly, LacIM1 and CI are only expressed at basal level. Without repression of the lamda promoter wild-type LacI is produced and represses the production of GFP. At the same point the N-Acyl homoserine lactone (AHL) Synthase LuxI is produced, AHL binds to LuxR which is constantly expressed and represses the red fluorescent protein (RFP). AHL is a quorum-sensing molecule with a high diffusion rate, it diffuses through the whole tube and represses RFP production in the whole tube, even in cells where no AHL is produced. If the xylene concentration is too high and it can not be degraded within the tube, no AHL is produced. Without AHL LuxR does not repress RFP and the whole tube turns red. To obtain better dynamics we tagged GFP and TetR with LVA tags. Finally we can also use a negative input for our system, in this case we have to introduce an additional inverter to invert the negative input signal into a positive one. Therefore the tetracycline repressor protein (TetR) was used in case of AlcR. If acetalydeyhde is present AlcR binds to the promotor of TetR and inhibit its represssion, resulting in no TetR.
|
Plasmid strategyTo variate the copy number of the different components of our system, we introduced 3 plasmid with different origin of replication in our system. One with pBR322 for high copy which has about 15-20 copies per cell, one p15A for medium copy with 10-12 copies per cell and a pSC101 for low copy numbers with 5 copies per cell.
|
References[1] [http://aem.asm.org/cgi/content/abstract/64/2/748 Sven Panke, Juan M. Sánchez-Romero, and Víctor de Lorenzo: Engineering of Quasi-Natural Pseudomonas putida Strains for Toluene Metabolism through an ortho-Cleavage Degradation Pathway, Appl Environ Microbiol, February 1998, 64: 748-751] [2] [http://www.nature.com/nature/journal/v434/n7037/full/nature03461.html Subhayu Basu, Yoram Gerchman1, Cynthia H. Collins, Frances H. Arnold & Ron Weiss: A synthetic multicellular system for programmed pattern formation, Nature 2005, 434: 1130-11342] |
 "
"