|
|
| (15 intermediate revisions not shown) |
| Line 19: |
Line 19: |
| | ======Promoters and Terminators====== | | ======Promoters and Terminators====== |
| | The iGEM registry is lacking in promoter and terminators sequences for yeast, especially when compared with the number available for use in ''E. coli''. In order to address this problem and increase the use of yeast in synthetic biology we have decided to provide a library of promoter and terminator sequences from 12 yeast genes: 4 causing high expression, 4 causing medium expression, and 4 causing low expression. We hope these BioBrick compatible parts will make it easier for future iGEM teams to work with ''Saccharomyces cerevisiae''. | | The iGEM registry is lacking in promoter and terminators sequences for yeast, especially when compared with the number available for use in ''E. coli''. In order to address this problem and increase the use of yeast in synthetic biology we have decided to provide a library of promoter and terminator sequences from 12 yeast genes: 4 causing high expression, 4 causing medium expression, and 4 causing low expression. We hope these BioBrick compatible parts will make it easier for future iGEM teams to work with ''Saccharomyces cerevisiae''. |
| | + | |
| | + | ======Characterizing the Promoters====== |
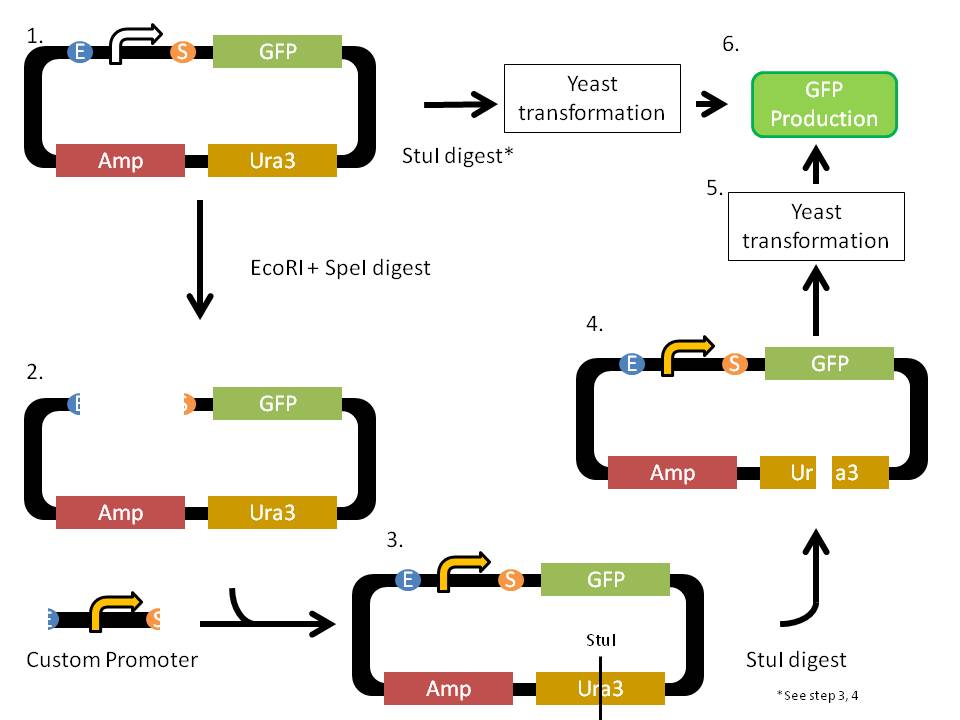
| | + | In order to provide useful parts with better ease of use, we have decided to not only provide a library of useful yeast promoters to the part registry, but also quantitatively characterize the strength of our promoters by using reference promoters. In a nut shell, we compared the ability of our promoters to express the GFP gene to that of other well characterized promoters in identical laboratory strain. This way, iGEM teams in the future can accurately predict the functionality of our promoters in their strains of interests by referencing the functionality of the reference promoters. A diagram and a brief step-by-step explanation of the process is provided below: |
| | + | |
| | + | [[File:Promoter_characterization_picture.jpg|center|610px]] |
| | + | |
| | + | |
| | + | # Plasmids with the reference promoter (in white), GFP, ampicilin (AMP) resistance, and Ura3 production genes were provided by the Tom Ellis lab in Imperial College London. Three different versions of the plasmids with different reference promoters (ADH1p, CYC1p, PFY1p) were provided. The AMP resistance gene allows for selection of bacterial transformation, while the URA3 production gene allows for selection of yeast transformation. |
| | + | #* The given plasmids were first transformed into ‘E. coli’ then miniprepped to retrieve the plasmids. Some of the miniprep product undergoes StuI digest (see step 4), while the rest undergoes EcoRI (E) and SpeI (S) digest. The E and S digest cleaves the reference promoter out of the plasmids. |
| | + | # The E + S digested plasmids were then ligated with our promoters of interest, which have also been digested with E + S to have compatible overhang. The ligation results in a complete plasmids with integrated promoters of interest |
| | + | # The complete plasmids then underwent StuI digest. The StuI digestion site is in the Ura3 gene. This step results in a linear DNA with parts of Ura3 gene on both ends. |
| | + | # The linear DNA from step 3 can then be integrated into the yeast genome due to the homologous Ura3 domains flanking the DNA. |
| | + | # The linear DNA pieces were then transformed into YPH500, a yeast strain that has a mutation in its Ura production gene. The transformed yeasts were then plated on Ura deficient YPD plates. Only cells with successful integration of linear DNA are able to grow. |
| | + | # Colonies were then picked and grown overnight. The GFP production of the cells was then analyzed using flow cytometry. The GFP production of strain with our promoters of interests was then compared to the GFP production of strains with the reference promoters. The relative strength of our promoters was quantified. |
| | | | |
| | ======Promoters====== | | ======Promoters====== |
| - | [[File:ConstitutivePromoter.png|link=http://partsregistry.org/Image:ConstitutivePromoter.png]] | + | [[File:Forward Constitutive Promoter.png|150px|link=http://www.sbolstandard.org/initiatives/sbol-visual]] |
| | | | |
| | Promoter sequences that have been BioBricked: | | Promoter sequences that have been BioBricked: |
| Line 35: |
Line 49: |
| | | | |
| | ======Terminators====== | | ======Terminators====== |
| - | [[File:Part icon terminator.png|322px|link=http://partsregistry.org/Image:Part_icon_terminator.png]] | + | [[File:Forward Terminator.png|150px|link=http://www.sbolstandard.org/initiatives/sbol-visual]] |
| | | | |
| | 3'UTR sequences that have been BioBricked: | | 3'UTR sequences that have been BioBricked: |
| Line 52: |
Line 66: |
| | ======Promoters and 3'UTRs for Golden Gate Assembly====== | | ======Promoters and 3'UTRs for Golden Gate Assembly====== |
| | We also have a second promoter/3'UTR library consisting of the same sequences with custom overhangs with BsaI sites rather than the BioBrick sites. These are being used in our two [[Team:Johns Hopkins/Project/Violacein|violacein projects]], where they will allow Golden Gate assembly of violacein expression cassettes. | | We also have a second promoter/3'UTR library consisting of the same sequences with custom overhangs with BsaI sites rather than the BioBrick sites. These are being used in our two [[Team:Johns Hopkins/Project/Violacein|violacein projects]], where they will allow Golden Gate assembly of violacein expression cassettes. |
| | + | |
| | | | |
| | <html> | | <html> |
| | </div> | | </div> |
Promoters and Terminators
The iGEM registry is lacking in promoter and terminators sequences for yeast, especially when compared with the number available for use in E. coli. In order to address this problem and increase the use of yeast in synthetic biology we have decided to provide a library of promoter and terminator sequences from 12 yeast genes: 4 causing high expression, 4 causing medium expression, and 4 causing low expression. We hope these BioBrick compatible parts will make it easier for future iGEM teams to work with Saccharomyces cerevisiae.
Characterizing the Promoters
In order to provide useful parts with better ease of use, we have decided to not only provide a library of useful yeast promoters to the part registry, but also quantitatively characterize the strength of our promoters by using reference promoters. In a nut shell, we compared the ability of our promoters to express the GFP gene to that of other well characterized promoters in identical laboratory strain. This way, iGEM teams in the future can accurately predict the functionality of our promoters in their strains of interests by referencing the functionality of the reference promoters. A diagram and a brief step-by-step explanation of the process is provided below:
- Plasmids with the reference promoter (in white), GFP, ampicilin (AMP) resistance, and Ura3 production genes were provided by the Tom Ellis lab in Imperial College London. Three different versions of the plasmids with different reference promoters (ADH1p, CYC1p, PFY1p) were provided. The AMP resistance gene allows for selection of bacterial transformation, while the URA3 production gene allows for selection of yeast transformation.
- The given plasmids were first transformed into ‘E. coli’ then miniprepped to retrieve the plasmids. Some of the miniprep product undergoes StuI digest (see step 4), while the rest undergoes EcoRI (E) and SpeI (S) digest. The E and S digest cleaves the reference promoter out of the plasmids.
- The E + S digested plasmids were then ligated with our promoters of interest, which have also been digested with E + S to have compatible overhang. The ligation results in a complete plasmids with integrated promoters of interest
- The complete plasmids then underwent StuI digest. The StuI digestion site is in the Ura3 gene. This step results in a linear DNA with parts of Ura3 gene on both ends.
- The linear DNA from step 3 can then be integrated into the yeast genome due to the homologous Ura3 domains flanking the DNA.
- The linear DNA pieces were then transformed into YPH500, a yeast strain that has a mutation in its Ura production gene. The transformed yeasts were then plated on Ura deficient YPD plates. Only cells with successful integration of linear DNA are able to grow.
- Colonies were then picked and grown overnight. The GFP production of the cells was then analyzed using flow cytometry. The GFP production of strain with our promoters of interests was then compared to the GFP production of strains with the reference promoters. The relative strength of our promoters was quantified.
Promoters
Promoter sequences that have been BioBricked:
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530016 RPS8Bp]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530005 BAP2p]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530015 FCY2p]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530018 Gal 1/10p]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530010 HHO1p]
|
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530003 KRE9p] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530007 PRY1p] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530004 STM1p] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530008 TDH3p] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530009 RPS2p]
|
Terminators
3'UTR sequences that have been BioBricked:
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530020 ARD1utr]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530006 BAP2utr]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530017 FCY2utr]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530019 HHo1utr]
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530014 KRE9utr]
|
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530013 PRY1utr] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530011 RPL8Autr] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530021 RPL24Autr] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530024 RPS2utr] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530022 RPS8Butr]
|
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K530023 TDH3utr] | | |
|
Promoters and 3'UTRs for Golden Gate Assembly
We also have a second promoter/3'UTR library consisting of the same sequences with custom overhangs with BsaI sites rather than the BioBrick sites. These are being used in our two violacein projects, where they will allow Golden Gate assembly of violacein expression cassettes.
 "
"