Team:Bielefeld-Germany/Results/S-Layer/Guide/3a
From 2011.igem.org

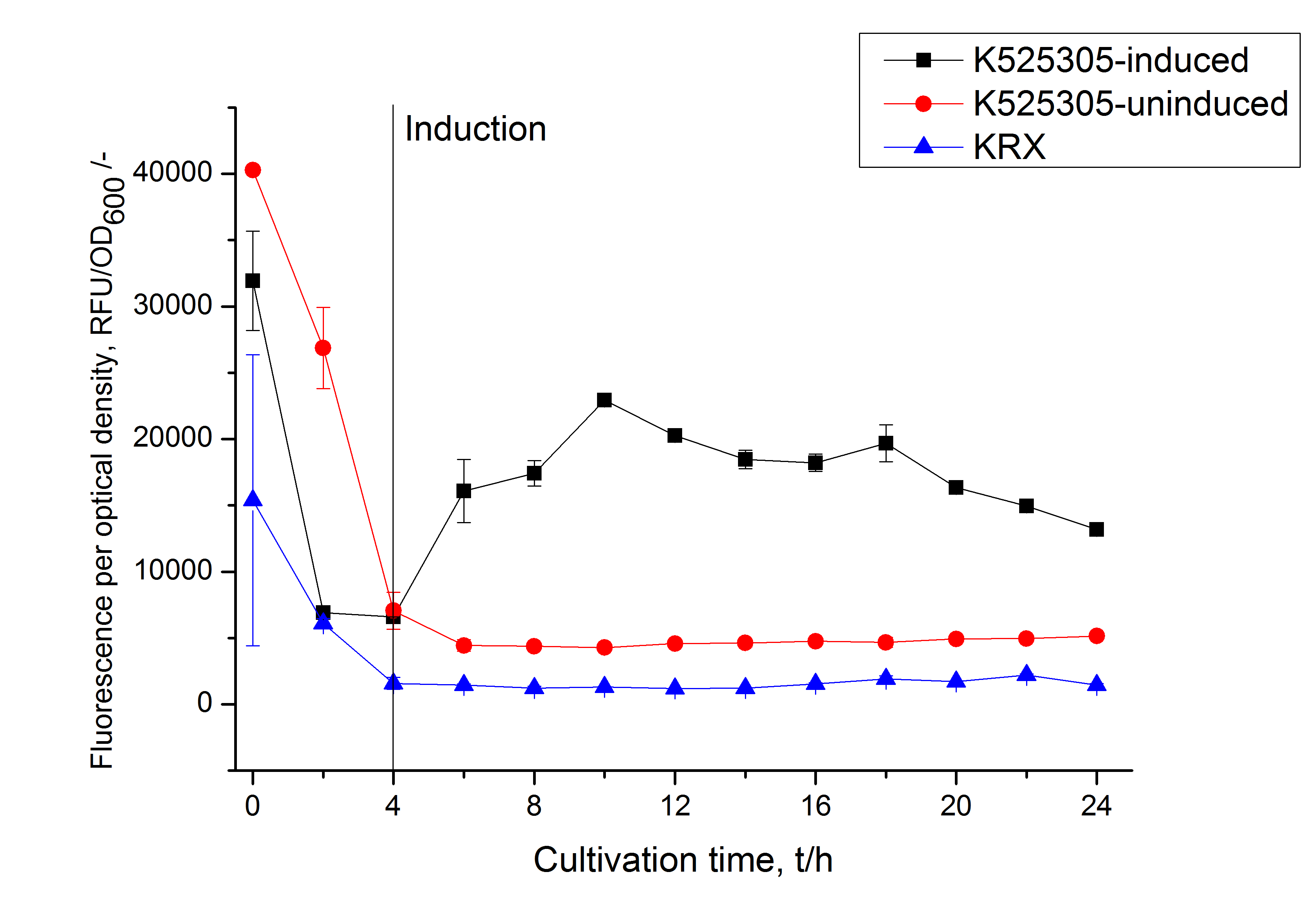
Easy and small scale expression of S-layer protein under control of T7 promoter
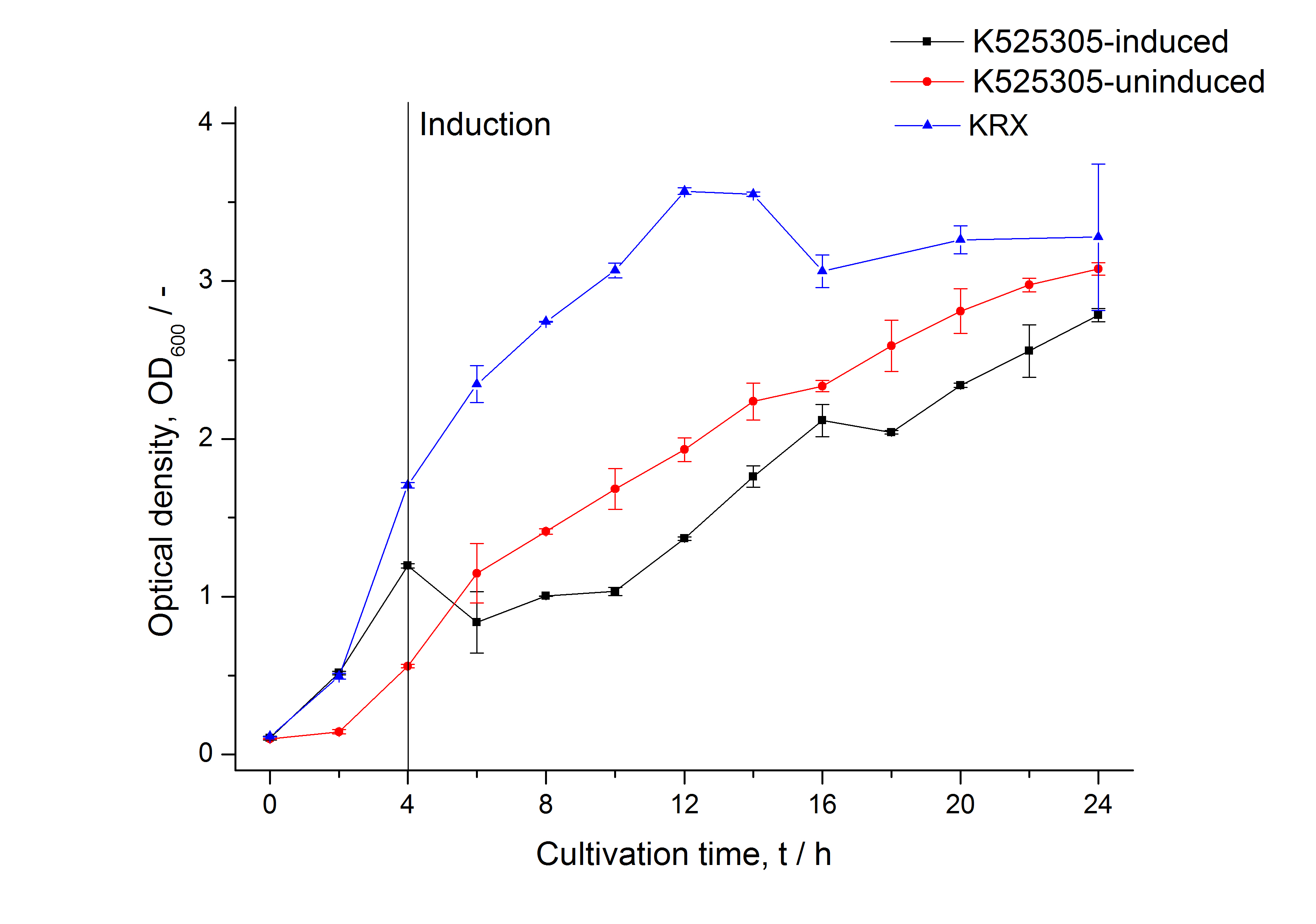
The expression of S-layer proteins is stressful for Escherichia coli. So using E. coli [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ KRX] to express genes under the control of a T7 promoter is an easy way to overexpress your proteins and seperate growth and production phase. This strain carries a T7 polymerase gene under the control of a rhamnose promoter in its genome and is also optimized for cloning so you do not have to transform your plasmids after assembly in e.g. TOP10, isolate them and bring them in an expression strain like BL21(DE3) - you can go with a single transformation step from assembly to production. The rhamnose promoter is a tightly controled promoter (compared to arabinose or lactose promoter) and is inhibited by glucose. Using glucose and L-rhamnose supplemented LB medium leads to an autoinduction of the rhamnose promoter when glucose is completely metabolized by the cells. L-rhamnose can not be metabolized by the cells but has the same effect like D-rhamnose on the rhamnose promoter.
Summarized: Use glucose, L-rhamnose and antibiotic supplemented LB medium, put it in a shaking flask, add your E. coli KRX cells carrying your S-layer fusion protein under the control of a T7 promoter, put the shaking flask on a shaker at 37 °C and then just wait! The cells will grow until the glucose is depleted (OD600 ~ 1 when inocculating with OD600 ~ 0.1) and the expression of the S-layer will start. Stop the cultivation after about 8 - 10 h and harvest your cells by centrifugation.
Want to know how to continue? Then read how to disrupt your cells here.
To show that this really works that easy: Look at the following figures of the expression of the fluorescent S-layer fusion protein SgsE | mCitrine using the autoinduction protocol.
 "
"

