Team:Bielefeld-Germany/Project/Background/S-Layer
From 2011.igem.org
(→S-layer) |
JSchwarzhans (Talk | contribs) (→References) |
||
| (16 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
=S-layer= | =S-layer= | ||
| - | [[Image:Bielefeld2011_enzyme_immobilization.png| | + | [[Image:Bielefeld2011_enzyme_immobilization.png|400px|right]] |
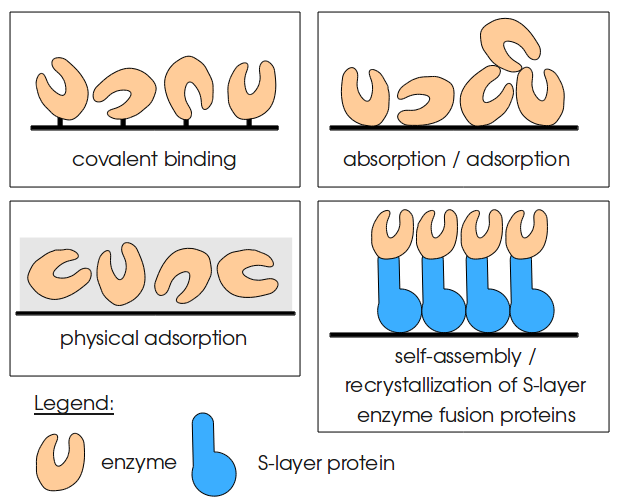
Molecular nanotechnology, especially nanobiotechnology, starts to use and modify functionalized surfaces. Particularly the immobilization of self-assembling biomolecules draws an increasing attention. The advantages of using immobilized enzymes in well-defined positions on nanostructured surfaces may even be greater. Whereas there exist various possibilities to immobilize enzymes like covalent binding, adsorption and physical adsorption, immobilization using S-layer enyzme fusion proteins provides and additional advantage due to the defined position of the enzymes. Therefore functional groups of enzymes can be immobilized in directed orientation (compare figure on the right). | Molecular nanotechnology, especially nanobiotechnology, starts to use and modify functionalized surfaces. Particularly the immobilization of self-assembling biomolecules draws an increasing attention. The advantages of using immobilized enzymes in well-defined positions on nanostructured surfaces may even be greater. Whereas there exist various possibilities to immobilize enzymes like covalent binding, adsorption and physical adsorption, immobilization using S-layer enyzme fusion proteins provides and additional advantage due to the defined position of the enzymes. Therefore functional groups of enzymes can be immobilized in directed orientation (compare figure on the right). | ||
| - | + | Self-assembly is the organization of molecules into defined structures, lowering the free energy of the system. Interaction between the molecules is non-covalent (''e.g.'' hydrophobic, van der Waals forces, molecular stacking) ([http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer ''et al.'', 2007]). | |
| - | + | ||
| - | Self-assembly is | + | |
Many biomolecules such as proteins, polysaccharides and lipids have the ability to self-assemble into different shapes (''e.g.'' spherical, rod- or sheet-like shapes), allowing several specific functions as virus capsids, cytoskeleton components or extracellular surface layer protein. | Many biomolecules such as proteins, polysaccharides and lipids have the ability to self-assemble into different shapes (''e.g.'' spherical, rod- or sheet-like shapes), allowing several specific functions as virus capsids, cytoskeleton components or extracellular surface layer protein. | ||
| - | The so-called paracrystalline cell surface- | + | The so-called paracrystalline cell surface-layers (S-layer) are built up from S-layer proteins and are one of the most common surface structures in bacteria and archaea. |
| + | They are regarded as the outmost cell envelope of prokaryotic organisms ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr ''et al.'', 2007]). | ||
| - | = | + | <br style="clear: both" /> |
| - | + | ==S-layers in general== | |
| - | [[Image:Bielefeld-Germany2011-S-Layer-Geometrien.jpg| | + | [[Image:Bielefeld-Germany2011-S-Layer-Geometrien.jpg|400px|right]] |
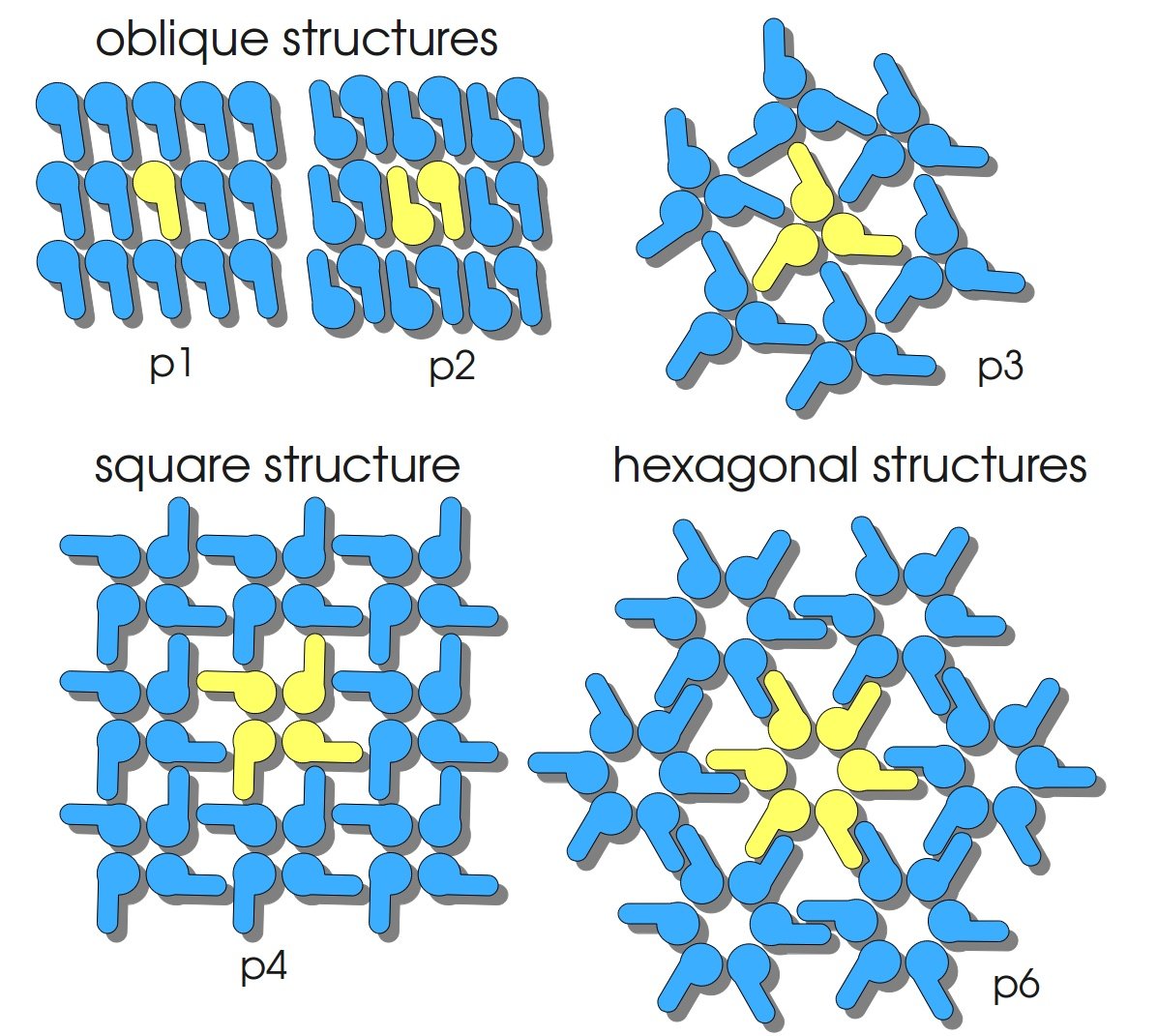
| - | + | S-layer proteins fulfill various functions as molecular sieves, ion traps and protective coats ([http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Sleytr ''et al.'', 2005]). They build up periodic structures, posses pores of identical size and morphology and show equal physicochemical properties on each molecular unit. Isolated they have the ability to reassemble into two-dimensional crystals equal to the S-layer structures that are found on intact bacterial cells. S-layers have the ability to form self-assembly products in solution and to recrystallize into monomolecular layers on solid supports, at air-water interface and on lipid films. They can completely cover liposomes and nanocapsules as well as small beads. S-layers are mainly composed of a single (glyco-)protein species, assembled into layers that are completely covering the outer part of the bacterial cell. In organisms they may represent up to 20 % of the total protein content of a bacterial cell. Most S-layer proteins are weakly acidic (pI 4 - 6) and contain a high proportion of hydrophobic amino acids as well as few or no sulphur-containing amino acids. Their molecular mass varies between 40 – 200 kDa and is often strain-specific. The assembled S-layer lattices in bacteria are generally 5 – 20 nm thick and in archaea up to 70 nm thick. The protein subunits of S-layers are arranged in lattices with different symmetry: oblique (p1, p2), square (p4) or hexagonal (p3, p6) with a center-to-center spacing of the subunits of 3 – 35 nm (compare figure on the right). S-layers are highly porous with a porosity of 30 – 70 % ([http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/abstract Sleytr ''et al.'', 2007]). | |
| - | + | <br style="clear: both" /> | |
| - | + | Various S-layer proteins from archaea and eubacteria are glycosylated, with strain-specific modifications. S-layer proteins were the first prokaryotic proteins that were shown to exhibit this characteristic. Until now, glycosylation has been proven for several archaeal S-layer proteins. Among the bacterial species glycosylation was demonstrated only for S-layer proteins of Bacillaceae ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami ''et al.'', 1997], [http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer ''et al.'', 2007]). | |
| + | In gram-negative bacteria, S-layer proteins are the exclusive cell wall component. In gram-positive bacteria and archaea, S-layers assemble on the outmost part of a firm wall matrix, which is composed mostly of peptidoglycan and pseudomurein. In gram-negative bacteria, S-layers are linked to specific lipopolysaccharides (LPS) ([http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Sleytr ''et al.'', 2005]). For gram-positive bacteria, a cell-wall-targeting domain could be identified at the N-terminal end of many S-layer proteins. The domain facilitates binding to a specific secondary cell wall polymer (SCWP) by a lectin-type binding ([http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/abstract Sleytr ''et al.'', 2007]). It was found that some S-layer proteins consist of two distinct domains with different functions. One domain is involved in the assembly with other S-layer protein monomers and the other domain mediates the interaction with the cell wall. Several SLH (S-layer homologous) domains have been identified at the amino-terminal region of different S-layer proteins and at the carboxy-terminal region of cell-associated exoproteins. The domain may be repeated within the sequence and is involved in anchoring the S-layer proteins to the cell surface ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami ''et al.'', 1997], [http://www.sciencedirect.com/science/article/pii/S0966842X99015139 Sleytr & Beveridge, 1999]). In various S-layer proteins from ''bacillacaea'' the deletion of significant parts of the carboxy-terminus or amino-terminus did not affect self-assembly and the capability of the S-layer proteins to form lattices ([http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/abstract Sleytr ''et al.'', 2007]). | ||
| + | The supramolecular structure as well as the mechanism of binding the outermost cell wall vary between S-layers of different species, leading to the development of different isolation procedures. S-layers normally are attached to the cell wall through non-covalent binding, and can therefore be isolated and completely disintegrated in dissociating agents (''e.g.'' lithium chloride), metal-chelating agents (''e.g.'' ethylendiaminetetraacetic acid EDTA), chaotropic denaturants such as urea or guanidine hydrochloride and by raising or lowering pH. After removal of the disrupting agent the reassembly takes place. ([http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Sleytr ''et al.'', 2005]). | ||
| - | ==The S-layer protein PS2 of '' | + | ==The S-layer protein PS2 of ''Corynebacterium glutamicum''== |
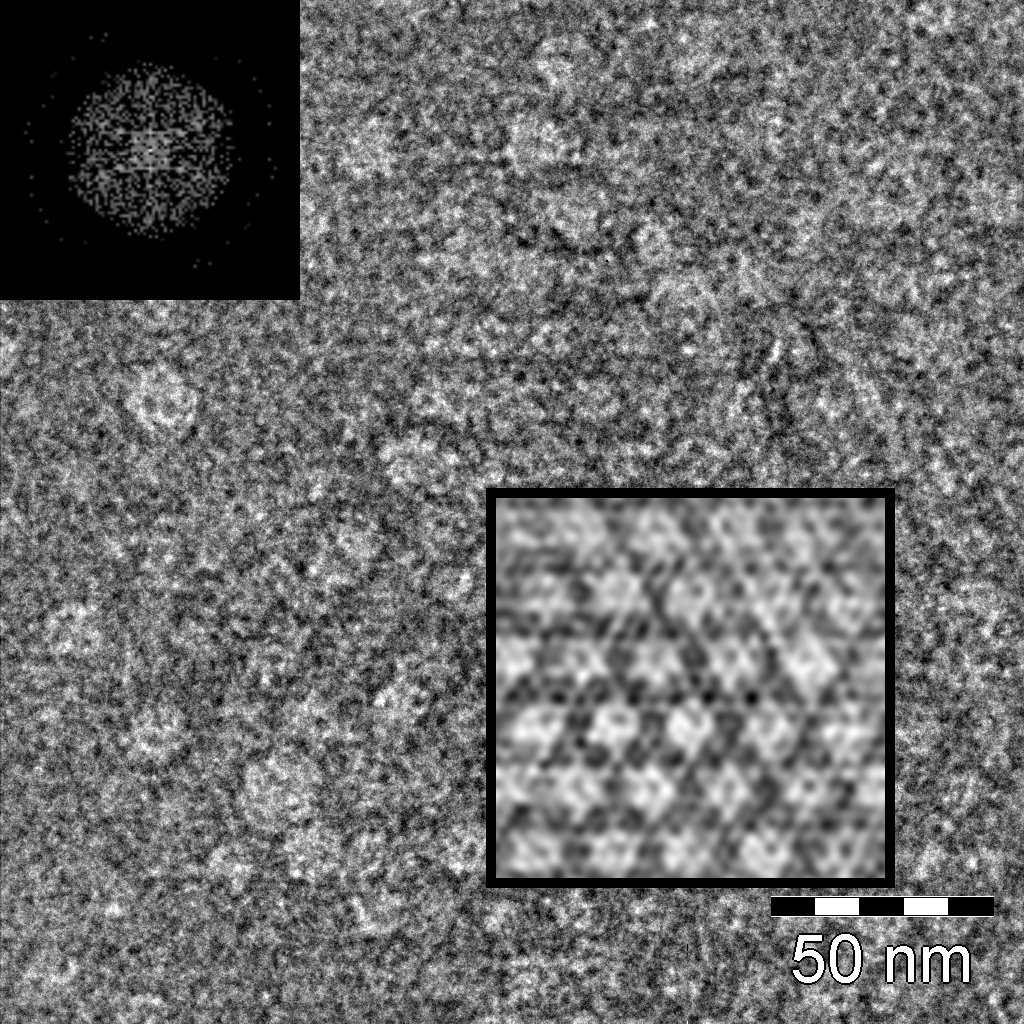
| - | The S-layer of the gram-positive bacterium ''Corynebacterium glutamicum'' ATCC 14067 is formed by the PS2 protein. The protein is encoded by the gene ''cspB''. The mature protein has a molecular mass of 52.5 kDa. It is devoid of any sulfur-containing amino acids, whereas its nature is due to a high content of hydrophobic amino acids. Although there exist a lot of different S-layer proteins, PS2 has no similarities to any other protein in the EMBL database. The S-layer of ''C. glutamicum'' is characterized by a hexagonal lattice symmetry. Attachment between S-layer and cell wall was found to be due to the hydrophobic carboxy-terminus of the PS2 protein. It was found that peptidoglycan is probably not involved in interaction between the PS2 S-layer and the cell because the interaction between PS2 and the cell is disrupted by adding detergents. Also the S-layer protein from ''C. glutamicum'' does not contain a SLH domain, which is characteristic for several S-layer proteins and other enzymes bound to the peptidoglycan. Besides some other S-layer proteins show a carboxy-terminal hydrophobic sequence of 20 – 24 amino acids. (''e.g. Halobacterium halobium, Haloferax volcanii, Rickettsia rickettsii'') ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami ''et al.'', 1997], [http://www.sciencedirect.com/science/article/pii/S016816560400241X Hansmeier ''et al.'', 2004]). | + | The S-layer of the gram-positive bacterium ''Corynebacterium glutamicum'' ATCC 14067 is formed by the PS2 protein. The protein is encoded by the gene ''cspB''. The mature protein has a molecular mass of 52.5 kDa. It is devoid of any sulfur-containing amino acids, whereas its hydrophobic nature is due to a high content of hydrophobic amino acids. Although there exist a lot of different S-layer proteins, PS2 has no similarities to any other protein in the EMBL database. The S-layer of ''C. glutamicum'' is characterized by a hexagonal lattice symmetry. Attachment between S-layer and cell wall was found to be due to the hydrophobic carboxy-terminus of the PS2 protein. It was found that peptidoglycan is probably not involved in interaction between the PS2 S-layer and the cell because the interaction between PS2 and the cell is disrupted by adding detergents. Also the S-layer protein from ''C. glutamicum'' does not contain a SLH domain, which is characteristic for several S-layer proteins and other enzymes bound to the peptidoglycan. Besides some other S-layer proteins show a carboxy-terminal hydrophobic sequence of 20 – 24 amino acids. (''e.g. Halobacterium halobium, Haloferax volcanii, Rickettsia rickettsii'') ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami ''et al.'', 1997], [http://www.sciencedirect.com/science/article/pii/S016816560400241X Hansmeier ''et al.'', 2004]). |
[[File:Bielefeld-Germany-TEM.jpg|300px|thumb|left|TEM Microscopy of PS2 with hexagonal lattice structure.]] | [[File:Bielefeld-Germany-TEM.jpg|300px|thumb|left|TEM Microscopy of PS2 with hexagonal lattice structure.]] | ||
| Line 42: | Line 43: | ||
SgsE monomers are naturally assembled in a lattice with oblique symmetry (p2) exhibiting a well-defined periodicity and distances of 9.4 – 11.6 nm between the proteinaceous subunits. The S-layer protein SgsE of ''Geobacillus stearothermophilus'' NRS 2004/3a consists of 903 amino acids, including a 30 amino acid signal peptide (SLH-domain) at the amino-terminus. | SgsE monomers are naturally assembled in a lattice with oblique symmetry (p2) exhibiting a well-defined periodicity and distances of 9.4 – 11.6 nm between the proteinaceous subunits. The S-layer protein SgsE of ''Geobacillus stearothermophilus'' NRS 2004/3a consists of 903 amino acids, including a 30 amino acid signal peptide (SLH-domain) at the amino-terminus. | ||
| - | The carboxy-terminal part of SgsE is the larger part of the protein, encoding the self-assembly information. The protein is formed by the ''sgsE'' gene, has a calculated mass of 93.7 kDa and an isoelectric point of 6.1. When isolated SgsE maintains its ability to self-assemble | + | The carboxy-terminal part of SgsE is the larger part of the protein, encoding the self-assembly information. The protein is formed by the ''sgsE'' gene, has a calculated mass of 93.7 kDa and an isoelectric point of 6.1. When isolated SgsE maintains its ability to self-assemble. Depending on salt concentration, duration of dialysis to remove the detergent and its amino acid sequence it builds up five types of self-assembly products. These products are formed like flat sheets and cylinders ([http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer ''et al.'', 2007]). |
==The S-layer protein SbpA of ''Lysinibacillus sphaericus'' CCM 2177== | ==The S-layer protein SbpA of ''Lysinibacillus sphaericus'' CCM 2177== | ||
| - | The S-layer protein SbpA of ''Lysinibacillus sphaericus'' CCM 2177 consists of 1,268 amino acids and is encoded by the ''sbpA'' gene. The lattice symmetry formed through self-assembly is a square structure (p4) with a center-to-center spacing of the morphological units of 13.1 nm. [http://pubs.acs.org/doi/abs/10.1021/bc800445r Badelt-Lichtblau ''et al.'' (2009)] found that the self-assembly process is strongly dependent on presence of bivalent cations. Therefore the assembly is dirigible, because in absence of bivalent cations SbpA stays water-soluble. At the amino-terminal end the protein consists of three typical SLH (S-layer homologous) domains and an additional 58 amino acid-long SLH-like motif. Those domains recognize a distinct type of secondary cell wall polymers (SCWP), consisting disaccharide units. The S-layer protein SbpA is extremely resistant to deletions changing its properties. The deletion of up to 237 carboxy-terminal amino acids does not affect the ability to self-assemble into square lattices. After deletion of 350 amino acids the lattice structure changes from square (p4) to oblique (p1) symmetry [http://pubs.acs.org/doi/abs/10.1021/bc800445r (Badelt-Lichtblau ''et al.'', 2009)]. | + | The S-layer protein SbpA of ''Lysinibacillus sphaericus'' CCM 2177 consists of 1,268 amino acids and is encoded by the ''sbpA'' gene. The lattice symmetry formed through self-assembly is a square structure (p4) with a center-to-center spacing of the morphological units of 13.1 nm. [http://pubs.acs.org/doi/abs/10.1021/bc800445r Badelt-Lichtblau ''et al.'' (2009)] found that the self-assembly process is strongly dependent on presence of bivalent cations. Therefore the assembly is dirigible, because in absence of bivalent cations SbpA stays water-soluble. At the amino-terminal end the protein consists of three typical SLH (S-layer homologous) domains and an additional 58 amino acid-long SLH-like motif. Those domains recognize a distinct type of secondary cell wall polymers (SCWP), consisting of disaccharide units. The S-layer protein SbpA is extremely resistant to deletions changing its properties. The deletion of up to 237 carboxy-terminal amino acids does not affect the ability to self-assemble into square lattices. After deletion of 350 amino acids the lattice structure changes from square (p4) to oblique (p1) symmetry [http://pubs.acs.org/doi/abs/10.1021/bc800445r (Badelt-Lichtblau ''et al.'', 2009)]. |
| - | + | ||
| - | + | ||
==Further applications of S-layer proteins== | ==Further applications of S-layer proteins== | ||
| Line 56: | Line 55: | ||
A current topic in S-layer research is the design of more sensitive and improved optical and electrochemical sensors. One example is the luminescence lifetime based oxygen sensor, described by [http://www.ncbi.nlm.nih.gov/pubmed/19765970 Schleicher ''et al.'' (2009)]. Therefore metalloporphyrin dyes which change luminescence properties in presence and absence of oxygen were bound to S-layer proteins. These proteins were used to form monolayer coatings on optical fiber surfaces allowing evanescent field fluorescence excitation of the linked fluorophores. The key benefits of using S-layer proteins for this type of sensor are the imparted anti-fouling properties and the good biocompatibility. This is especially valuable for sensing applications in complex biological fluids or implantable sensors. | A current topic in S-layer research is the design of more sensitive and improved optical and electrochemical sensors. One example is the luminescence lifetime based oxygen sensor, described by [http://www.ncbi.nlm.nih.gov/pubmed/19765970 Schleicher ''et al.'' (2009)]. Therefore metalloporphyrin dyes which change luminescence properties in presence and absence of oxygen were bound to S-layer proteins. These proteins were used to form monolayer coatings on optical fiber surfaces allowing evanescent field fluorescence excitation of the linked fluorophores. The key benefits of using S-layer proteins for this type of sensor are the imparted anti-fouling properties and the good biocompatibility. This is especially valuable for sensing applications in complex biological fluids or implantable sensors. | ||
| - | Concentrating on the repetitive features of S-layer proteins to form a well defined nano lattice, the use as filtration units | + | Concentrating on the repetitive features of S-layer proteins to form a well defined nano lattice, the use as filtration units becomes reasonable. [http://jb.asm.org/cgi/content/full/182/4/859 Sára and Sleytr (2000)] described a so-called isoporous S-layer ultrafiltration membrane (SUM). This filtration system is based on a nylon microfiltration membrane as recrystallization surface for S-layer fragments of ''Bacillus sphaericus'' CCM 2120, to build up a constant lattice with a well-defined pore size of 12.5 nm. |
Another advantage of this membrane is the presence of functional groups in defined positions and orientations. These functional groups could be used for highly reproducible chemical modifications to optimize molecular sieving properties and non-specific adsorption (antifouling) characteristics. | Another advantage of this membrane is the presence of functional groups in defined positions and orientations. These functional groups could be used for highly reproducible chemical modifications to optimize molecular sieving properties and non-specific adsorption (antifouling) characteristics. | ||
| Line 89: | Line 88: | ||
Sleytr UB, Sára M, Pum D, Schuster B, Messner P, Schäffer C (2005) Self-assembling protein systems: microbial S-layers, in: [http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Steinbüchel A, Fahnestock SR (Eds.), Polyamides 34 and complex proteinaceous materials, Wiley-VCH, Weinheim, pp. 285-338]. | Sleytr UB, Sára M, Pum D, Schuster B, Messner P, Schäffer C (2005) Self-assembling protein systems: microbial S-layers, in: [http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Steinbüchel A, Fahnestock SR (Eds.), Polyamides 34 and complex proteinaceous materials, Wiley-VCH, Weinheim, pp. 285-338]. | ||
| - | Sleytr UB, Egelseer EM, Ilk N, Pum D, Schuster B (2007) S-Layers as a basic building block in a molecular construction kit, [http://onlinelibrary.wiley.com/doi/10.1111/j. | + | Sleytr UB, Egelseer EM, Ilk N, Pum D, Schuster B (2007) S-Layers as a basic building block in a molecular construction kit, [http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/full FEBS J 274(2):323-34]. |
Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full FEMS Microbiol Lett. 267(2):131-44]. | Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full FEMS Microbiol Lett. 267(2):131-44]. | ||
Völlenkle C, Weigert S, Ilk N et al. (2004) Construction of a functional S-layer fusion protein comprising an immunoglobulin G-binding domain for development of specific adsorbents for extracorporeal blood purification,[http://aem.asm.org/cgi/content/short/70/3/1514 Appl Environ Microbiol 70: 1514–1521]. | Völlenkle C, Weigert S, Ilk N et al. (2004) Construction of a functional S-layer fusion protein comprising an immunoglobulin G-binding domain for development of specific adsorbents for extracorporeal blood purification,[http://aem.asm.org/cgi/content/short/70/3/1514 Appl Environ Microbiol 70: 1514–1521]. | ||
Latest revision as of 01:53, 29 October 2011


Contents |
S-layer
Molecular nanotechnology, especially nanobiotechnology, starts to use and modify functionalized surfaces. Particularly the immobilization of self-assembling biomolecules draws an increasing attention. The advantages of using immobilized enzymes in well-defined positions on nanostructured surfaces may even be greater. Whereas there exist various possibilities to immobilize enzymes like covalent binding, adsorption and physical adsorption, immobilization using S-layer enyzme fusion proteins provides and additional advantage due to the defined position of the enzymes. Therefore functional groups of enzymes can be immobilized in directed orientation (compare figure on the right).
Self-assembly is the organization of molecules into defined structures, lowering the free energy of the system. Interaction between the molecules is non-covalent (e.g. hydrophobic, van der Waals forces, molecular stacking) ([http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer et al., 2007]). Many biomolecules such as proteins, polysaccharides and lipids have the ability to self-assemble into different shapes (e.g. spherical, rod- or sheet-like shapes), allowing several specific functions as virus capsids, cytoskeleton components or extracellular surface layer protein. The so-called paracrystalline cell surface-layers (S-layer) are built up from S-layer proteins and are one of the most common surface structures in bacteria and archaea.
They are regarded as the outmost cell envelope of prokaryotic organisms ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
S-layers in general
S-layer proteins fulfill various functions as molecular sieves, ion traps and protective coats ([http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Sleytr et al., 2005]). They build up periodic structures, posses pores of identical size and morphology and show equal physicochemical properties on each molecular unit. Isolated they have the ability to reassemble into two-dimensional crystals equal to the S-layer structures that are found on intact bacterial cells. S-layers have the ability to form self-assembly products in solution and to recrystallize into monomolecular layers on solid supports, at air-water interface and on lipid films. They can completely cover liposomes and nanocapsules as well as small beads. S-layers are mainly composed of a single (glyco-)protein species, assembled into layers that are completely covering the outer part of the bacterial cell. In organisms they may represent up to 20 % of the total protein content of a bacterial cell. Most S-layer proteins are weakly acidic (pI 4 - 6) and contain a high proportion of hydrophobic amino acids as well as few or no sulphur-containing amino acids. Their molecular mass varies between 40 – 200 kDa and is often strain-specific. The assembled S-layer lattices in bacteria are generally 5 – 20 nm thick and in archaea up to 70 nm thick. The protein subunits of S-layers are arranged in lattices with different symmetry: oblique (p1, p2), square (p4) or hexagonal (p3, p6) with a center-to-center spacing of the subunits of 3 – 35 nm (compare figure on the right). S-layers are highly porous with a porosity of 30 – 70 % ([http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/abstract Sleytr et al., 2007]).
Various S-layer proteins from archaea and eubacteria are glycosylated, with strain-specific modifications. S-layer proteins were the first prokaryotic proteins that were shown to exhibit this characteristic. Until now, glycosylation has been proven for several archaeal S-layer proteins. Among the bacterial species glycosylation was demonstrated only for S-layer proteins of Bacillaceae ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami et al., 1997], [http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer et al., 2007]).
In gram-negative bacteria, S-layer proteins are the exclusive cell wall component. In gram-positive bacteria and archaea, S-layers assemble on the outmost part of a firm wall matrix, which is composed mostly of peptidoglycan and pseudomurein. In gram-negative bacteria, S-layers are linked to specific lipopolysaccharides (LPS) ([http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Sleytr et al., 2005]). For gram-positive bacteria, a cell-wall-targeting domain could be identified at the N-terminal end of many S-layer proteins. The domain facilitates binding to a specific secondary cell wall polymer (SCWP) by a lectin-type binding ([http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/abstract Sleytr et al., 2007]). It was found that some S-layer proteins consist of two distinct domains with different functions. One domain is involved in the assembly with other S-layer protein monomers and the other domain mediates the interaction with the cell wall. Several SLH (S-layer homologous) domains have been identified at the amino-terminal region of different S-layer proteins and at the carboxy-terminal region of cell-associated exoproteins. The domain may be repeated within the sequence and is involved in anchoring the S-layer proteins to the cell surface ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami et al., 1997], [http://www.sciencedirect.com/science/article/pii/S0966842X99015139 Sleytr & Beveridge, 1999]). In various S-layer proteins from bacillacaea the deletion of significant parts of the carboxy-terminus or amino-terminus did not affect self-assembly and the capability of the S-layer proteins to form lattices ([http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/abstract Sleytr et al., 2007]).
The supramolecular structure as well as the mechanism of binding the outermost cell wall vary between S-layers of different species, leading to the development of different isolation procedures. S-layers normally are attached to the cell wall through non-covalent binding, and can therefore be isolated and completely disintegrated in dissociating agents (e.g. lithium chloride), metal-chelating agents (e.g. ethylendiaminetetraacetic acid EDTA), chaotropic denaturants such as urea or guanidine hydrochloride and by raising or lowering pH. After removal of the disrupting agent the reassembly takes place. ([http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Sleytr et al., 2005]).
The S-layer protein PS2 of Corynebacterium glutamicum
The S-layer of the gram-positive bacterium Corynebacterium glutamicum ATCC 14067 is formed by the PS2 protein. The protein is encoded by the gene cspB. The mature protein has a molecular mass of 52.5 kDa. It is devoid of any sulfur-containing amino acids, whereas its hydrophobic nature is due to a high content of hydrophobic amino acids. Although there exist a lot of different S-layer proteins, PS2 has no similarities to any other protein in the EMBL database. The S-layer of C. glutamicum is characterized by a hexagonal lattice symmetry. Attachment between S-layer and cell wall was found to be due to the hydrophobic carboxy-terminus of the PS2 protein. It was found that peptidoglycan is probably not involved in interaction between the PS2 S-layer and the cell because the interaction between PS2 and the cell is disrupted by adding detergents. Also the S-layer protein from C. glutamicum does not contain a SLH domain, which is characteristic for several S-layer proteins and other enzymes bound to the peptidoglycan. Besides some other S-layer proteins show a carboxy-terminal hydrophobic sequence of 20 – 24 amino acids. (e.g. Halobacterium halobium, Haloferax volcanii, Rickettsia rickettsii) ([http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Chami et al., 1997], [http://www.sciencedirect.com/science/article/pii/S016816560400241X Hansmeier et al., 2004]).
The S-layer protein SgsE of Geobacillus stearothermophilus NRS 2004/3a
SgsE monomers are naturally assembled in a lattice with oblique symmetry (p2) exhibiting a well-defined periodicity and distances of 9.4 – 11.6 nm between the proteinaceous subunits. The S-layer protein SgsE of Geobacillus stearothermophilus NRS 2004/3a consists of 903 amino acids, including a 30 amino acid signal peptide (SLH-domain) at the amino-terminus. The carboxy-terminal part of SgsE is the larger part of the protein, encoding the self-assembly information. The protein is formed by the sgsE gene, has a calculated mass of 93.7 kDa and an isoelectric point of 6.1. When isolated SgsE maintains its ability to self-assemble. Depending on salt concentration, duration of dialysis to remove the detergent and its amino acid sequence it builds up five types of self-assembly products. These products are formed like flat sheets and cylinders ([http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Schäffer et al., 2007]).
The S-layer protein SbpA of Lysinibacillus sphaericus CCM 2177
The S-layer protein SbpA of Lysinibacillus sphaericus CCM 2177 consists of 1,268 amino acids and is encoded by the sbpA gene. The lattice symmetry formed through self-assembly is a square structure (p4) with a center-to-center spacing of the morphological units of 13.1 nm. [http://pubs.acs.org/doi/abs/10.1021/bc800445r Badelt-Lichtblau et al. (2009)] found that the self-assembly process is strongly dependent on presence of bivalent cations. Therefore the assembly is dirigible, because in absence of bivalent cations SbpA stays water-soluble. At the amino-terminal end the protein consists of three typical SLH (S-layer homologous) domains and an additional 58 amino acid-long SLH-like motif. Those domains recognize a distinct type of secondary cell wall polymers (SCWP), consisting of disaccharide units. The S-layer protein SbpA is extremely resistant to deletions changing its properties. The deletion of up to 237 carboxy-terminal amino acids does not affect the ability to self-assemble into square lattices. After deletion of 350 amino acids the lattice structure changes from square (p4) to oblique (p1) symmetry [http://pubs.acs.org/doi/abs/10.1021/bc800445r (Badelt-Lichtblau et al., 2009)].
Further applications of S-layer proteins
The ability of self-assembly and forming defined nanostructures enables the use of S-layer proteins as building blocks in a biomolecular construction kit for the generation of a universal nanobiotechnological matrix. By using the matrix as a nano pinboard for further components or just the nanostructure itself, a great variety of applications in modular nanobiotechnology, biomimetics, bioanalytics and medical diagnostic are imaginable [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full (Sleytr et al., 2007)].
A current topic in S-layer research is the design of more sensitive and improved optical and electrochemical sensors. One example is the luminescence lifetime based oxygen sensor, described by [http://www.ncbi.nlm.nih.gov/pubmed/19765970 Schleicher et al. (2009)]. Therefore metalloporphyrin dyes which change luminescence properties in presence and absence of oxygen were bound to S-layer proteins. These proteins were used to form monolayer coatings on optical fiber surfaces allowing evanescent field fluorescence excitation of the linked fluorophores. The key benefits of using S-layer proteins for this type of sensor are the imparted anti-fouling properties and the good biocompatibility. This is especially valuable for sensing applications in complex biological fluids or implantable sensors.
Concentrating on the repetitive features of S-layer proteins to form a well defined nano lattice, the use as filtration units becomes reasonable. [http://jb.asm.org/cgi/content/full/182/4/859 Sára and Sleytr (2000)] described a so-called isoporous S-layer ultrafiltration membrane (SUM). This filtration system is based on a nylon microfiltration membrane as recrystallization surface for S-layer fragments of Bacillus sphaericus CCM 2120, to build up a constant lattice with a well-defined pore size of 12.5 nm. Another advantage of this membrane is the presence of functional groups in defined positions and orientations. These functional groups could be used for highly reproducible chemical modifications to optimize molecular sieving properties and non-specific adsorption (antifouling) characteristics.
The self-assembly and immobilization properties of S-layer fusion proteins also open new innovative perspectives in clinical applications like immune therapy, blood purification and drug targeting. [http://www.jimmunol.org/content/172/11/6642.full Bohle et al. (2004)] showed that a genetic fusion of an allergen to SbsC S-layer proteins from Geobacillus stearothermophilus combine reduced allergenicity with immunomodulatory capacity. These qualities make them to an ideal allergen carrier/adjuvants in specific immunotherapy, which is the only causative treatment for type I allergy. Fusion proteins of a synthetic analogue of the immunoglobulin G (IgG)-binding B-domain of protein A of Staphylococcus aureus and SbpA S-layer proteins from Bacillus sphaericus CCM 2177 are proposed as a microsphere-based extracorporeal blood detoxification system to remove IgG from human plasma from patients suffering from autoimmune disease. The use of microparticles to detoxify the plasma allows rapid removal of the pathogenic substances and leads to higher binding capacities than commercially available immunoadsorbents [http://aem.asm.org/cgi/content/short/70/3/1514 (Völlenkle et al., 2003)]. The natural/original ability of crystallization on lipid membranes/liposomes for heightening the mechanical and thermal stability of S-liposomes and the possibility for immobilizing biologically active molecules can be cross-linked and exploited as a matrix for the covalent attachment of functional molecules to built up a biocompatible drug targeting or gene therapy system.
But the main advantage of S-layer proteins is their application as universal matrices for the immobilization. This versatility could be upgraded by fusing the S-layers with minimum-sized core-streptavidin to build up a universal affinity matrix for any kind of biotinylated molecule. This concept fits exactly in the primary aim of constructing a biomolecular construction kit. [http://onlinelibrary.wiley.com/doi/10.1002/smll.200500147/abstract Huber et al. (2006)] used the streptavidin matrix for building up functional sensor surface by recrystallization of these heterotetramers on gold chips. This could be exploited for the development of cheaper DNA or protein chips.
In conclusion, S-layer proteins are one of the most innovative and promising discoveries for combining biology with engineering in the new field of nanobiotechnology.
References
Badelt-Lichtblau H, Kainz B, Völlenkle C, Egelseer EM, Sleytr UB, Pum D, Ilk N (2009) Genetic engineering of the S-layer protein SbpA of Lysinibacillus sphaericus CCM 2177 for the generation of functionalized nanoarrays, [http://pubs.acs.org/doi/abs/10.1021/bc800445r Bioconjug Chem. 20(5):895-903].
Bohle B, Breitwieser A, Zwölfer B, Jahn-Schmid B, S´ara M, Sleytr UB & Ebner C (2004) A novel approach to specific allergy treatment: the recombinant fusion protein of a bacterial cell surface (S-layer) protein and the major birch pollen allergen Bet v 1 (rSbsC-Bet v 1) combines reduced allergenicity with immunomodulating capacity, [http://www.jimmunol.org/content/172/11/6642.full J Immunol 172: 6642–6648].
Chami M, Bayan N, Peyret JL, Gulik-Krzywicki T, Leblon G, Shechter E (1997) The S-layer protein of Corynebacterium glutamicum is anchored to the cell wall by its C-terminal hydrophobic domain, [http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.d01-1868.x/abstract Mol Microbiol. 23(3):483-92].
Hansmeier N, Bartels FW, Ros R, Anselmetti D, Tauch A, Pühler A, Kalinowski J (2004) Classification of hyper-variable Corynebacterium glutamicum surface-layer proteins by sequence analyses and atomic force microscopy, [http://www.sciencedirect.com/science/article/pii/S016816560400241X J Biotechnol. 26;112(1-2):177-93].
Huber C, Liu J, Egelseer EM, Moll D, Knoll W, Sleytr UB & S´ara M (2006b) Heterotetramers formed by an S-layer-streptavidin fusion protein and core-streptavidin as nanoarrayed template for biochip development, [http://onlinelibrary.wiley.com/doi/10.1002/smll.200500147/abstract Small 2: 142–150].
Sára M and Sleytr U B (2000) S-layer proteins: A minireview, [http://jb.asm.org/cgi/content/full/182/4/859 Journal of bacteriology 182(4): 859–868].
Scheicher SR, Kainz B, Köstler S, Suppan M, Bizzarri A, Pum D, Sleytr UB, Ribitsch V (2009) Optical oxygen sensors based on Pt(II) porphyrin dye immobilized on S-layer protein matrices, [http://www.ncbi.nlm.nih.gov/pubmed/19765970 Biosensors and Bioelectronics 25: 797–802].
Schäffer C, Novotny R, Küpcü S, Zayni S, Scheberl A, Friedmann J, Sleytr UB, Messner P (2007) Novel biocatalysts based on S-layer self-assembly of Geobacillus stearothermophilus NRS 2004/3a: a nanobiotechnological approach, [http://onlinelibrary.wiley.com/doi/10.1002/smll.200700200/abstract Small 3(9):1549-59].
Sleytr UB, Beveridge TJ (1999) Bacterial S-layers. [http://www.sciencedirect.com/science/article/pii/S0966842X99015139 Trends Microbiol. 7(6):253-60].
Sleytr UB, Sára M, Pum D, Schuster B, Messner P, Schäffer C (2005) Self-assembling protein systems: microbial S-layers, in: [http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol7011/abstract Steinbüchel A, Fahnestock SR (Eds.), Polyamides 34 and complex proteinaceous materials, Wiley-VCH, Weinheim, pp. 285-338].
Sleytr UB, Egelseer EM, Ilk N, Pum D, Schuster B (2007) S-Layers as a basic building block in a molecular construction kit, [http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2006.05606.x/full FEBS J 274(2):323-34].
Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full FEMS Microbiol Lett. 267(2):131-44].
Völlenkle C, Weigert S, Ilk N et al. (2004) Construction of a functional S-layer fusion protein comprising an immunoglobulin G-binding domain for development of specific adsorbents for extracorporeal blood purification,[http://aem.asm.org/cgi/content/short/70/3/1514 Appl Environ Microbiol 70: 1514–1521].
 "
"