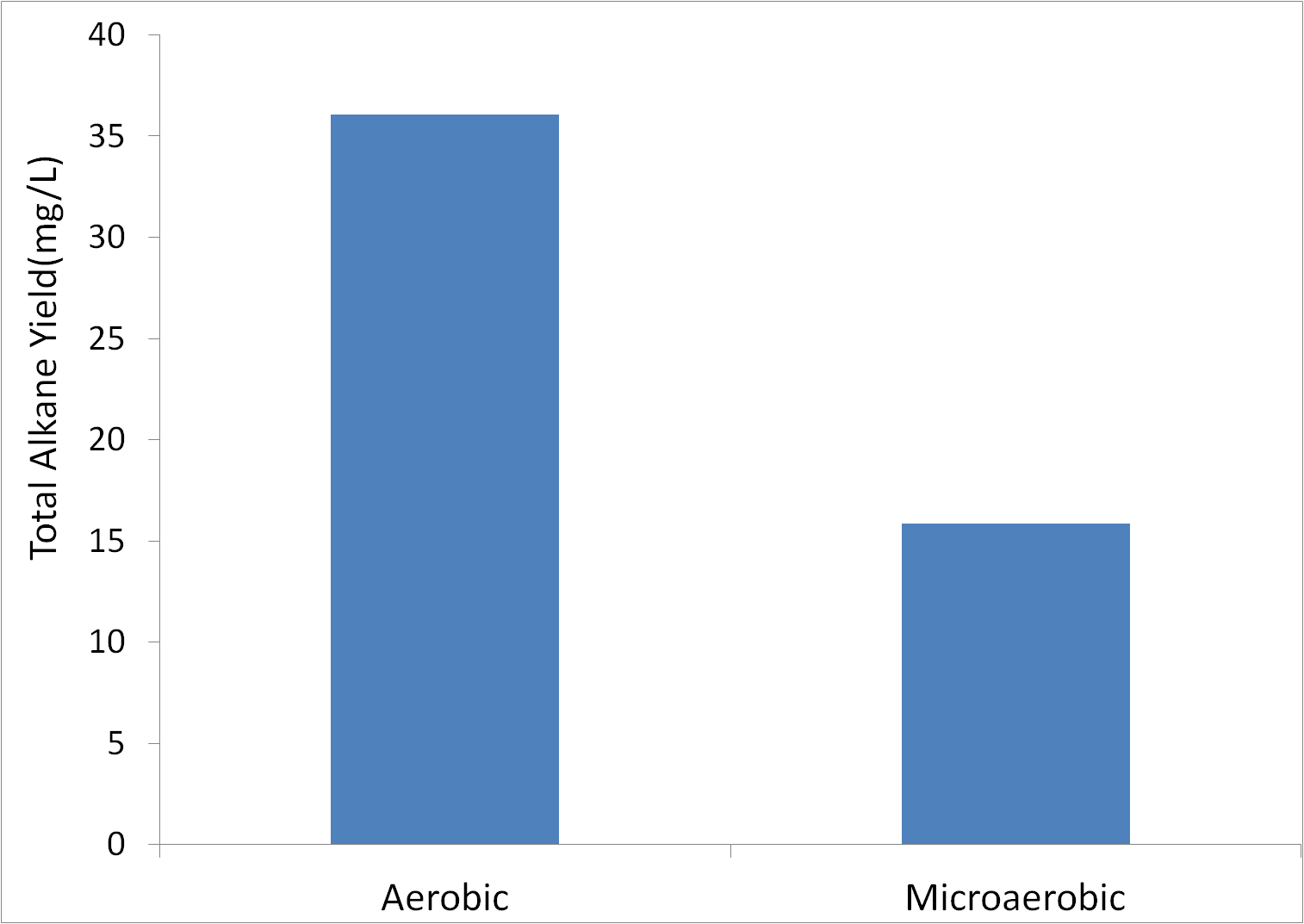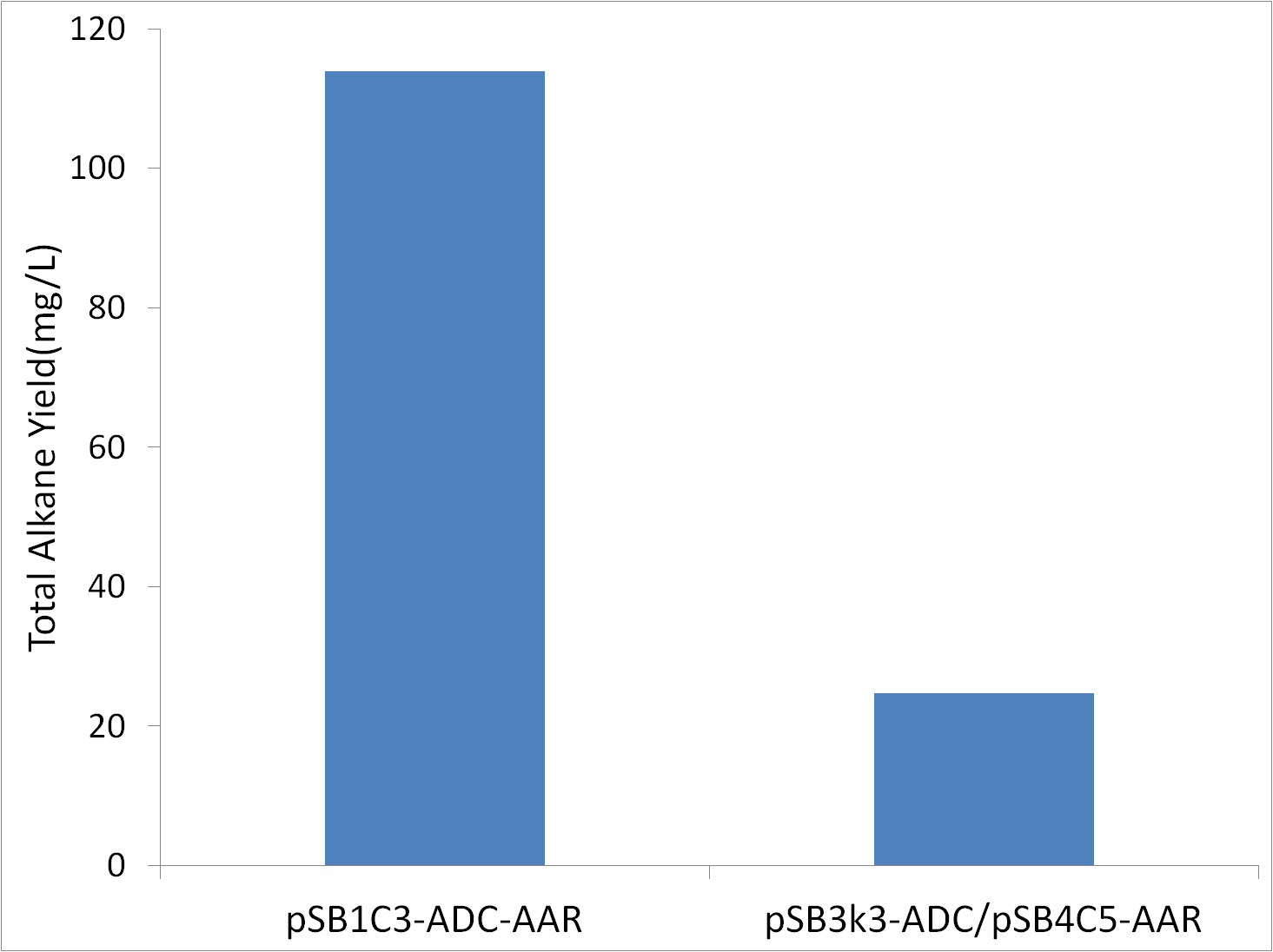Team:Washington/Alkanes/Future/Vector
From 2011.igem.org
(→'References) |
|||
| (19 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
| - | ='''System Optimization'''= | + | ='''<center>System Optimization</center>'''= |
Using our initial, non-optimized growth conditions, we were able to obtain alkane/ene yields of approximately 2 mg/L. In order to make analysis easier, and to make it easier to determine the effects of the addition of additional modules, we wanted to increase yield. Our efforts focused on how by varying system conditions, we could increase yield. | Using our initial, non-optimized growth conditions, we were able to obtain alkane/ene yields of approximately 2 mg/L. In order to make analysis easier, and to make it easier to determine the effects of the addition of additional modules, we wanted to increase yield. Our efforts focused on how by varying system conditions, we could increase yield. | ||
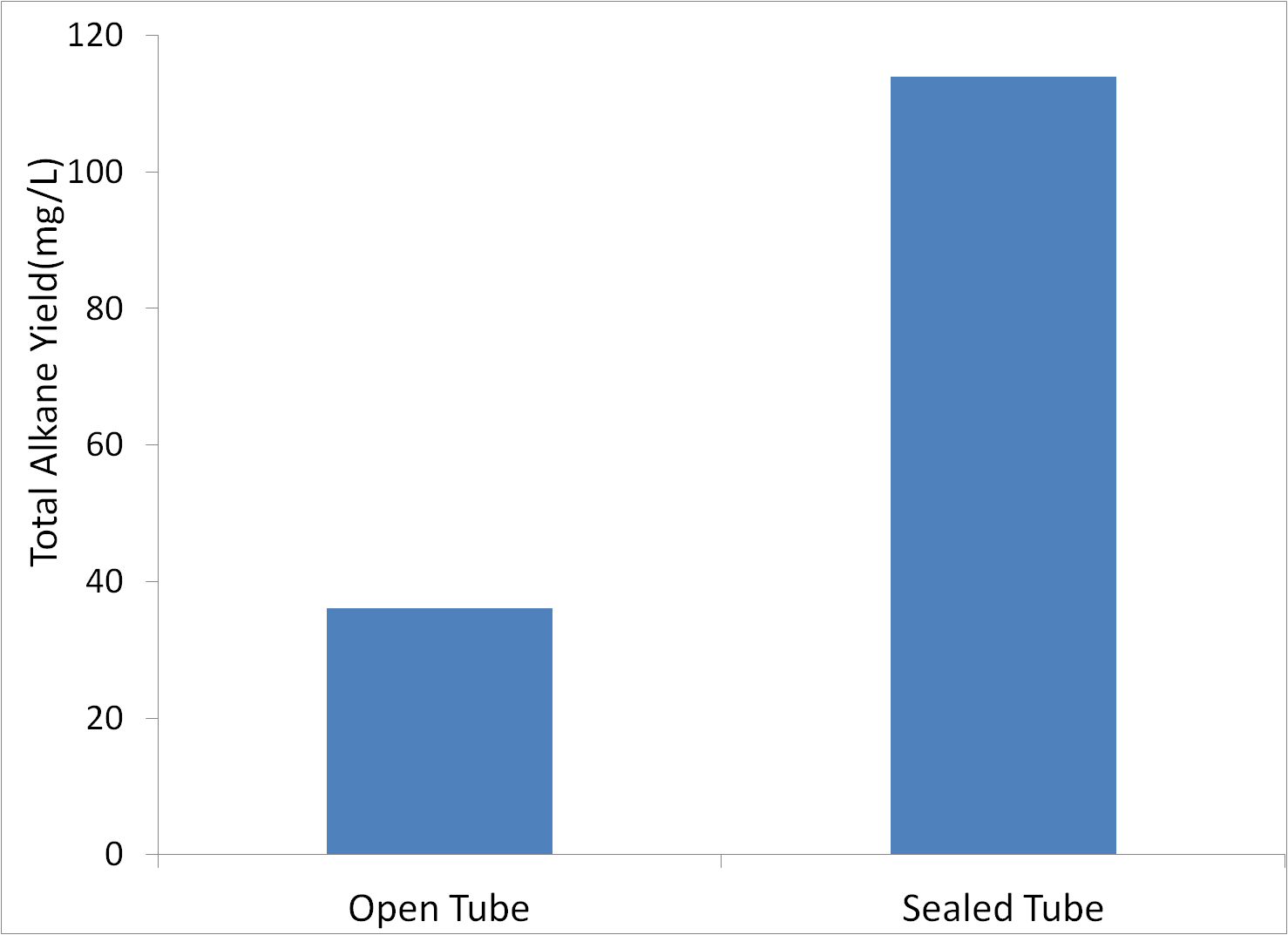
=='''Sealed vs. Open Tubes'''== | =='''Sealed vs. Open Tubes'''== | ||
| - | We were concerned that | + | We were concerned that produced alkanes may have been evaporating, reducing apparent yield. Therefore, we performed tests where the tubes were either capped the standard way, or capped with foil coverting the opening, thereby reducing evaporation. Tests were conducted in glass tubes with M9 production media, with MG1655 innoculated to an initial OD600 of 1. |
[[File:Washinton_open_sealed.png|center|500px|thumb|Sealing Culture Tubes Increases Yield.]] | [[File:Washinton_open_sealed.png|center|500px|thumb|Sealing Culture Tubes Increases Yield.]] | ||
| - | Covered tubes showed significantly more | + | Covered tubes showed significantly more alkanes, so all further tests would be conducted in sealed tubes. |
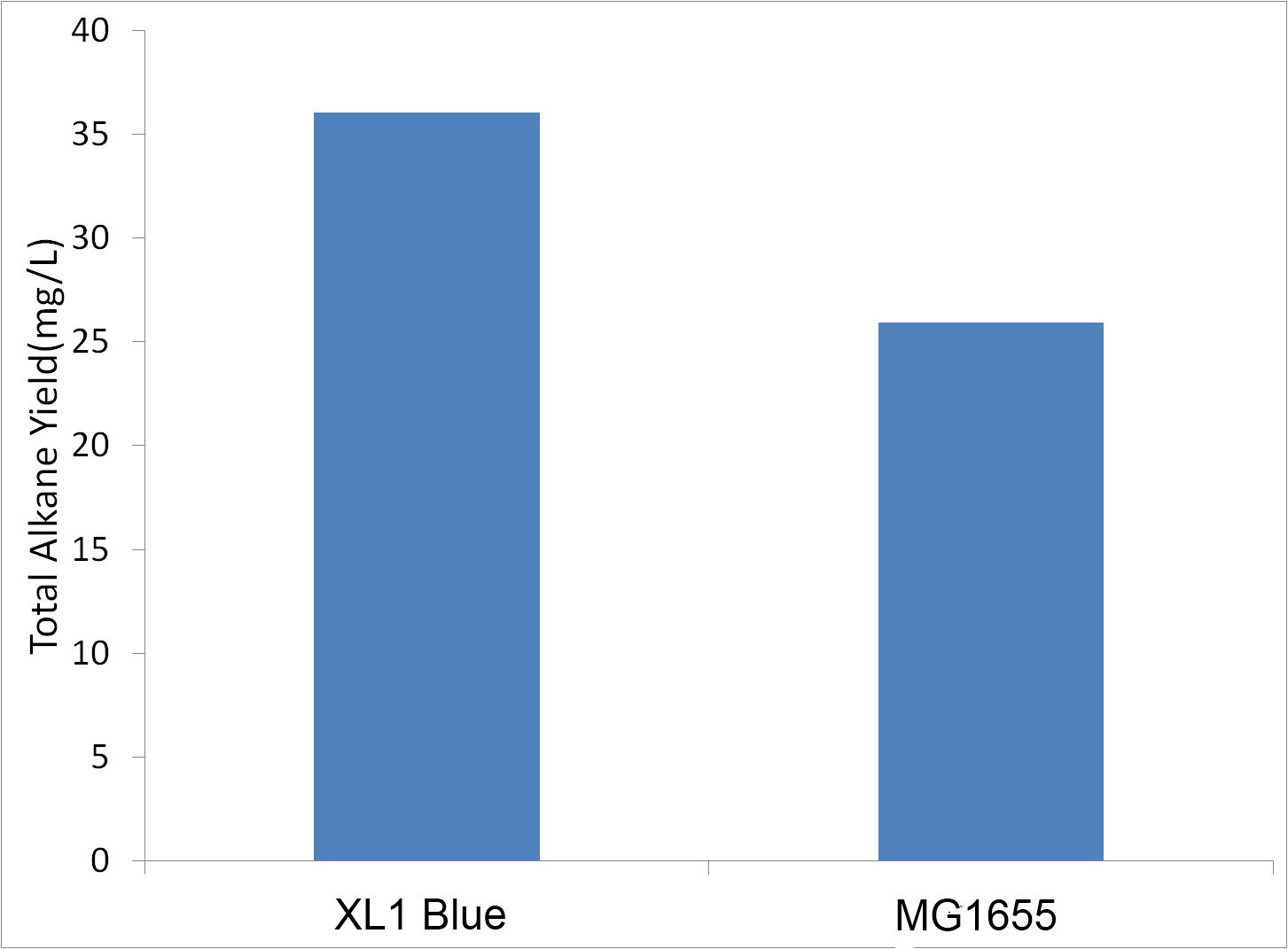
=='''Use of Different Strains'''== | =='''Use of Different Strains'''== | ||
| - | We had suspected that different strains of ''E. coli'' would produce varying amounts of | + | We had suspected that different strains of ''E. coli'' would produce varying amounts of alkanes. Initial experiments were done in MG1655, and we decided to test XL-1 blue (a commercial supercopentent variant of DH5a) for the ability to produce alkanes. Tests were conducted in sealed glass culture tubes in M9 production media . Cells were innoculated to an OD600 of 1 when conducting this test. |
[[File:Washinton_XL21vsMG1655.png|center|500px|thumb|XL-1 Blue Produces more Alkane than MG1655]] | [[File:Washinton_XL21vsMG1655.png|center|500px|thumb|XL-1 Blue Produces more Alkane than MG1655]] | ||
| - | XL-1 blue was able to produce more | + | XL-1 blue was able to produce more alkanes than MG1655. Therefore, future tests were conducted in XL-1 Blue. |
=='''Aerobic vs Microaerobic Growth'''== | =='''Aerobic vs Microaerobic Growth'''== | ||
| - | In many industrial production applications, growth in conditions with little or no oxygen can improve yield. Therefore, we tested alkane yield in cultures grown in airtight vials with only a small amount of air on the top in order to severely limit oxygen availability. Tests were conducted in glass( note that the test was conducted in open culture tubes for aerobic, | + | In many industrial production applications, growth in conditions with little or no oxygen can improve yield. Therefore, we tested alkane yield in cultures grown in airtight vials with only a small amount of air on the top in order to severely limit oxygen availability. Tests were conducted in glass (note that the test was conducted in open culture tubes for aerobic cultures, and sealed glass vials for microaerobic cultures, with M9 production media in XL21-blue cells.) |
[[File:Washington_aerobicVsMicroaerobic.png|center|500px|thumb|Oxygen has a Positive Effect on Alkane Production]] | [[File:Washington_aerobicVsMicroaerobic.png|center|500px|thumb|Oxygen has a Positive Effect on Alkane Production]] | ||
| - | Based upon this data, aerobic growth appears to be better for alkane yield. Note that this difference is not likely due to differences in growth rate, as both cultures were inoculated to a high OD600 of 1. The fact that this tube was not covered means that the actual alkane yield( before evaporation) was likely higher. Therefore, microaerobic conditions are unlikely to improve yield. | + | Based upon this data, aerobic growth appears to be better for alkane yield. Note that this difference is not likely due to differences in growth rate, as both cultures were inoculated to a high OD600 of 1. The fact that this tube was not covered means that the actual alkane yield (before evaporation) was likely higher. Therefore, microaerobic conditions are unlikely to improve yield. |
=='''pSB1C3-ADC-AAR vs pSB3k3-ADC/pSB4C5-AAR'''== | =='''pSB1C3-ADC-AAR vs pSB3k3-ADC/pSB4C5-AAR'''== | ||
| - | The original study expressed ADC and AAR on seperate low copy number vectors. If we could get high alkane yields using low copy number vectors, the lesser protien and vector production would make the use of a lower copy number vector preferable. Tests were conducted in covered glass culture tubes(aerobic conditions) with M9 production media. Cells were XL1-Blue, and were innoculated to an OD600 of 1. | + | The original study expressed ADC and AAR on seperate low copy number vectors. If we could get high alkane yields using low copy number vectors, the lesser protien and vector production would make the use of a lower copy number vector preferable. Tests were conducted in covered glass culture tubes (aerobic conditions) with M9 production media. Cells were XL1-Blue, and were innoculated to an OD600 of 1. |
[[File:Washington_copynumber.png|center|500px|thumb|Greater ADC/AAR Expression Results in More Alkane Production.]] | [[File:Washington_copynumber.png|center|500px|thumb|Greater ADC/AAR Expression Results in More Alkane Production.]] | ||
The fact that high copynumber vector use results in an increase in yield means that high expression levels of ADC and/or AAR are required for high alkane yields. | The fact that high copynumber vector use results in an increase in yield means that high expression levels of ADC and/or AAR are required for high alkane yields. | ||
=='''Media composition'''== | =='''Media composition'''== | ||
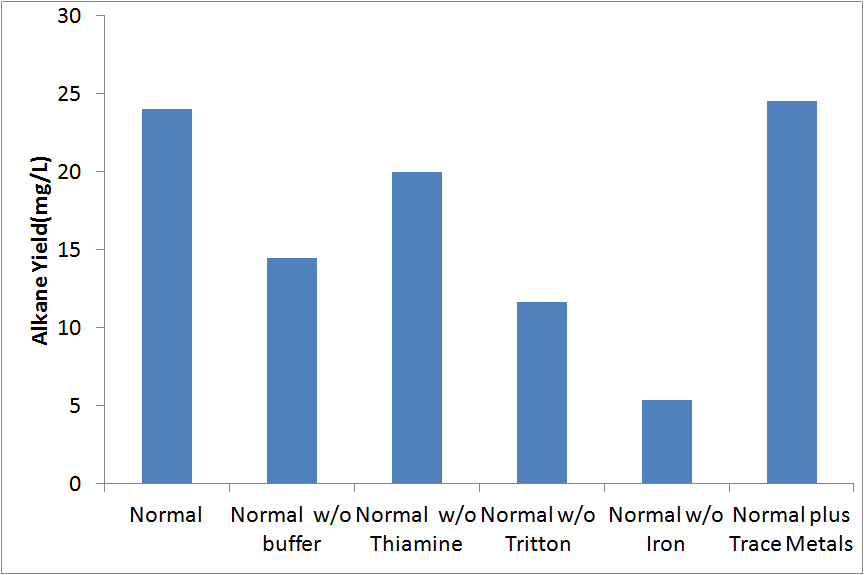
| - | Our initial media was based upon the media used in | + | Our initial media was based upon the media used in [[#References |[1]]]. We did not know if all of the additional components not found in a normal media (buffer, thiamine, Triton, iron) actually improved yield. In order to determine if each of these components imporved yield, we analyzed cultures inoculated into M9 medias without each of these 4 compounds. In addition, we tested media with additional trace metals. These tests were conducted in covered glass culture tubes with XL-1 blue cells. |
[[File:Washington_mediaconditions.png|center|500px|thumb|Effects of Media Composition on Alkane Yield.]] | [[File:Washington_mediaconditions.png|center|500px|thumb|Effects of Media Composition on Alkane Yield.]] | ||
None of the modifications we made resulted in a significant increase in yield. Therefore, no further modifications to the media used was made. | None of the modifications we made resulted in a significant increase in yield. Therefore, no further modifications to the media used was made. | ||
=='''Effects of Changing Initial Cell Density'''== | =='''Effects of Changing Initial Cell Density'''== | ||
| - | Earlier tests were done with an initial OD600 of 1. However, we had never established what the optimal initial OD would be. If significant alkane production occurs during stationary phase, inoculation to a high initial OD may increase alkane production. For this test, we inoculated XL-1 blue( to a final OD600 in a range of 0.01 to 10) in M9 production media( in covered glass culture tubes). We extracted from these cultures after 16 hours of growth. | + | Earlier tests were done with an initial OD600 of 1. However, we had never established what the optimal initial OD would be. If significant alkane production occurs during stationary phase, inoculation to a high initial OD may increase alkane production. For this test, we inoculated XL-1 blue (to a final OD600 in a range of 0.01 to 10) in M9 production media (in covered glass culture tubes). We extracted from these cultures after 16 hours of growth. |
[[File:Washington_alkaneODcurve.png|center|500px|thumb|Greater Initial Cell Density Results in Higher Alkane Yield.]] | [[File:Washington_alkaneODcurve.png|center|500px|thumb|Greater Initial Cell Density Results in Higher Alkane Yield.]] | ||
| - | Higher cell density cultures showed significantly higher alkane yields than lower cell density cultures. The increase of alkane yields at cell densities as high as 10 implies that high amounts of alkane production occurs during stationary phase. Therefore, alkane production scales with cell density. In addition, | + | Higher cell density cultures showed significantly higher alkane yields than lower cell density cultures. The increase of alkane yields at cell densities as high as 10 implies that high amounts of alkane production occurs during stationary phase. Therefore, alkane production scales with cell density. In addition, inducing alkane production during stationary phase decreases the amount of glucose going towards cellular growth instead of alkane production. |
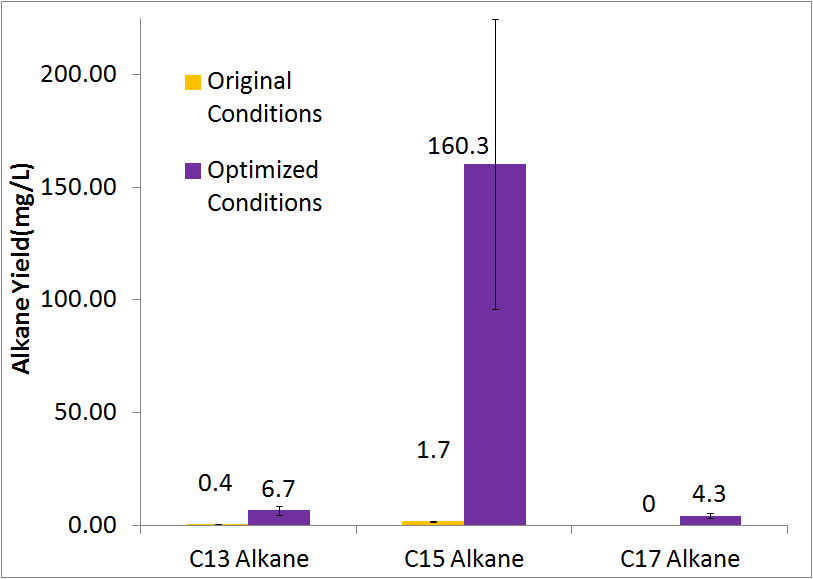
| + | =='''Final conditions'''== | ||
| + | Using our optimized media & growth conditions(XL-1 blue inoculated to an OD600 of 10, in M9 media, in closed glass culture tubes) , we were able to improve total alkane yield 80 fold, from approximenly 2 mg/L (after 48 hrs) to approximently 170 mg/L(after 48 hrs). | ||
| + | [[File:Washinton 2011 Optimization Quant.png|center|550px|thumb|Our optimized growth conditions resulted in an 80 fold increase in total alkane yield.]] | ||
| + | =='''Production Curve'''== | ||
| + | Ideally, alkane extraction and analysis would occur after all ( or essentially all) of the glucose in the media was used up. However, we didn't know how long it would take for our cells to use up all of the glucose. In order to determine the best time for extraction, we extracted alkanes from M9 production cultures that had been producing for 6, 24, 48, and 72 hours. Three cultures were used for each timepoint. | ||
| + | [[file:Washington_Productioncurve.png|center|550px|thumb|Total alkane yield over time under optimized conditions.]] | ||
| + | The 24 hour timepoint was essentially identical to the 48 and 72 hour timepoints. This implies that our cells are using up 30g/L glucose sometime before 24 hours. Future work will consist of increasing glucose concentration, and increasing inoculated cell density in order to maximize alkane production per culture per day. | ||
| + | =='''Alternate Carbon Sources'''== | ||
| + | While previous tests were done using glucose as the sole carbon source, it would be helpful to be able to use carbon sources that can be considered waste sources. There is a large amount of interest in using non-simple sugar carbon sources, such as cellulosic biomass, and even using carbon dioxide directly. However, these carbon sources cannot be easily utilized by ''E. coli''. Glycerol is a 3-carbon triol that is a waste product in the production of current generation biodiesel(fatty acid methyl esters(FAMEs). Glycerol can be used by ''E. coli'' as a sole carbon source, so we tried to use the PetroBrick to convert glycerol into diesel. We performed standard alkane production/analysis, with the second production media being either [https://2011.igem.org/Team:Washington/alkanebiosynthesis#Current_Protocol_for_100mL_of_Media standard M9 glucose media], or M9 media varients containing an equal mass of glycerol instead of glucose, or media without any carbon source. | ||
| + | [[File:Washington_carbontest.png|center|550px|thumb|Total alkane yield in M9 glucose, M9 glycerol, and M9 nedia without carbon.]] | ||
| + | The glucose media resulted in higher total alkane yield than the glycerol media(240 mg/L vs. 56.3 mg/L), but this may be due to media conditions being optimized for glucose as a carbon source insteam od glycerol. Further optimization may be able to improve on alkane yield when using glycerol as a carbon source. In media without carbon, we observed negligable alkane production(4.7 mg/L). This indicates that alkane production is due to conversion of carbon in production media into alkanes, not due to the conversion of cellular biomass into alkanes. This means that initial growth for cell density can occur in a rich media without having any major influence on yield. | ||
=='''References'''== | =='''References'''== | ||
Microbial Biosynthesis of Alkanes Andreas Schirmer, Mathew A. Rude, Xuezhi Li, Emanuela Popova and Stephen B. del Cardayre Science 30 July 2010: Vol. 329 no. 5991 pp. 559-562 http://www.sciencemag.org/content/329/5991/559 | Microbial Biosynthesis of Alkanes Andreas Schirmer, Mathew A. Rude, Xuezhi Li, Emanuela Popova and Stephen B. del Cardayre Science 30 July 2010: Vol. 329 no. 5991 pp. 559-562 http://www.sciencemag.org/content/329/5991/559 | ||
Latest revision as of 20:27, 20 November 2011
System Optimization
Using our initial, non-optimized growth conditions, we were able to obtain alkane/ene yields of approximately 2 mg/L. In order to make analysis easier, and to make it easier to determine the effects of the addition of additional modules, we wanted to increase yield. Our efforts focused on how by varying system conditions, we could increase yield.
Sealed vs. Open Tubes
We were concerned that produced alkanes may have been evaporating, reducing apparent yield. Therefore, we performed tests where the tubes were either capped the standard way, or capped with foil coverting the opening, thereby reducing evaporation. Tests were conducted in glass tubes with M9 production media, with MG1655 innoculated to an initial OD600 of 1.
Covered tubes showed significantly more alkanes, so all further tests would be conducted in sealed tubes.
Use of Different Strains
We had suspected that different strains of E. coli would produce varying amounts of alkanes. Initial experiments were done in MG1655, and we decided to test XL-1 blue (a commercial supercopentent variant of DH5a) for the ability to produce alkanes. Tests were conducted in sealed glass culture tubes in M9 production media . Cells were innoculated to an OD600 of 1 when conducting this test.
XL-1 blue was able to produce more alkanes than MG1655. Therefore, future tests were conducted in XL-1 Blue.
Aerobic vs Microaerobic Growth
In many industrial production applications, growth in conditions with little or no oxygen can improve yield. Therefore, we tested alkane yield in cultures grown in airtight vials with only a small amount of air on the top in order to severely limit oxygen availability. Tests were conducted in glass (note that the test was conducted in open culture tubes for aerobic cultures, and sealed glass vials for microaerobic cultures, with M9 production media in XL21-blue cells.)
Based upon this data, aerobic growth appears to be better for alkane yield. Note that this difference is not likely due to differences in growth rate, as both cultures were inoculated to a high OD600 of 1. The fact that this tube was not covered means that the actual alkane yield (before evaporation) was likely higher. Therefore, microaerobic conditions are unlikely to improve yield.
pSB1C3-ADC-AAR vs pSB3k3-ADC/pSB4C5-AAR
The original study expressed ADC and AAR on seperate low copy number vectors. If we could get high alkane yields using low copy number vectors, the lesser protien and vector production would make the use of a lower copy number vector preferable. Tests were conducted in covered glass culture tubes (aerobic conditions) with M9 production media. Cells were XL1-Blue, and were innoculated to an OD600 of 1.
The fact that high copynumber vector use results in an increase in yield means that high expression levels of ADC and/or AAR are required for high alkane yields.
Media composition
Our initial media was based upon the media used in [1]. We did not know if all of the additional components not found in a normal media (buffer, thiamine, Triton, iron) actually improved yield. In order to determine if each of these components imporved yield, we analyzed cultures inoculated into M9 medias without each of these 4 compounds. In addition, we tested media with additional trace metals. These tests were conducted in covered glass culture tubes with XL-1 blue cells.
None of the modifications we made resulted in a significant increase in yield. Therefore, no further modifications to the media used was made.
Effects of Changing Initial Cell Density
Earlier tests were done with an initial OD600 of 1. However, we had never established what the optimal initial OD would be. If significant alkane production occurs during stationary phase, inoculation to a high initial OD may increase alkane production. For this test, we inoculated XL-1 blue (to a final OD600 in a range of 0.01 to 10) in M9 production media (in covered glass culture tubes). We extracted from these cultures after 16 hours of growth.
Higher cell density cultures showed significantly higher alkane yields than lower cell density cultures. The increase of alkane yields at cell densities as high as 10 implies that high amounts of alkane production occurs during stationary phase. Therefore, alkane production scales with cell density. In addition, inducing alkane production during stationary phase decreases the amount of glucose going towards cellular growth instead of alkane production.
Final conditions
Using our optimized media & growth conditions(XL-1 blue inoculated to an OD600 of 10, in M9 media, in closed glass culture tubes) , we were able to improve total alkane yield 80 fold, from approximenly 2 mg/L (after 48 hrs) to approximently 170 mg/L(after 48 hrs).
Production Curve
Ideally, alkane extraction and analysis would occur after all ( or essentially all) of the glucose in the media was used up. However, we didn't know how long it would take for our cells to use up all of the glucose. In order to determine the best time for extraction, we extracted alkanes from M9 production cultures that had been producing for 6, 24, 48, and 72 hours. Three cultures were used for each timepoint.
The 24 hour timepoint was essentially identical to the 48 and 72 hour timepoints. This implies that our cells are using up 30g/L glucose sometime before 24 hours. Future work will consist of increasing glucose concentration, and increasing inoculated cell density in order to maximize alkane production per culture per day.
Alternate Carbon Sources
While previous tests were done using glucose as the sole carbon source, it would be helpful to be able to use carbon sources that can be considered waste sources. There is a large amount of interest in using non-simple sugar carbon sources, such as cellulosic biomass, and even using carbon dioxide directly. However, these carbon sources cannot be easily utilized by E. coli. Glycerol is a 3-carbon triol that is a waste product in the production of current generation biodiesel(fatty acid methyl esters(FAMEs). Glycerol can be used by E. coli as a sole carbon source, so we tried to use the PetroBrick to convert glycerol into diesel. We performed standard alkane production/analysis, with the second production media being either standard M9 glucose media, or M9 media varients containing an equal mass of glycerol instead of glucose, or media without any carbon source.
The glucose media resulted in higher total alkane yield than the glycerol media(240 mg/L vs. 56.3 mg/L), but this may be due to media conditions being optimized for glucose as a carbon source insteam od glycerol. Further optimization may be able to improve on alkane yield when using glycerol as a carbon source. In media without carbon, we observed negligable alkane production(4.7 mg/L). This indicates that alkane production is due to conversion of carbon in production media into alkanes, not due to the conversion of cellular biomass into alkanes. This means that initial growth for cell density can occur in a rich media without having any major influence on yield.
References
Microbial Biosynthesis of Alkanes Andreas Schirmer, Mathew A. Rude, Xuezhi Li, Emanuela Popova and Stephen B. del Cardayre Science 30 July 2010: Vol. 329 no. 5991 pp. 559-562 http://www.sciencemag.org/content/329/5991/559
 "
"